乳酸铵与正丁醇酯化反应动力学
IF 1.5
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
作为乳酸和聚乳酸生产新集成技术的一个阶段,首次研究了在130–170°C的温度范围内,在水的存在下,乳酸铵与正丁醇在封闭系统中相互作用生产乳酸丁酯的动力学。考虑到内酰胺的副生成和乳酸对酯化的酸催化作用,首次提出了乳酸铵与正丁醇酯化的反应方案和动力学模型。所开发的动力学模型可用于乳酸丁酯合成反应器的数学建模。本文章由计算机程序翻译,如有差异,请以英文原文为准。
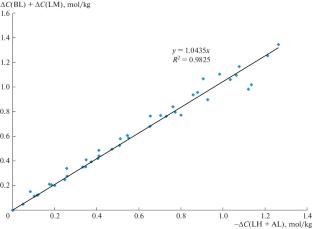
Kinetics of Esterification of Ammonium Lactate with n-Butanol
The kinetics of butyl lactate production by the interaction of ammonium lactate with n-butanol in the presence of water in the temperature range of 130–170°C in a closed system was studied for the first time as one of the stages of a new integrated technology for the production of lactic acid and polylactide. Also for the first time, a reaction scheme and kinetic model of ammonium lactate esterification with n-butanol were proposed, taking into account the side formation of lactamide and the acid catalysis of esterification by lactic acid. The developed kinetic model can be used for mathematical modeling of a reactor for the synthesis of butyl lactate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Physical Chemistry
化学-物理化学
CiteScore
1.50
自引率
0.00%
发文量
9
审稿时长
6-12 weeks
期刊介绍:
Doklady Physical Chemistry is a monthly journal containing English translations of current Russian research in physical chemistry from the Physical Chemistry sections of the Doklady Akademii Nauk (Proceedings of the Russian Academy of Sciences). The journal publishes the most significant new research in physical chemistry being done in Russia, thus ensuring its scientific priority. Doklady Physical Chemistry presents short preliminary accounts of the application of the state-of-the-art physical chemistry ideas and methods to the study of organic and inorganic compounds and macromolecules; polymeric, inorganic and composite materials as well as corresponding processes. The journal is intended for scientists in all fields of chemistry and in interdisciplinary sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


