具有突变(R22A)的视网膜胍基环化酶激活蛋白5 (GCAP5)的化学移位分配,该突变消除了二聚化并增强了环化酶的激活
IF 0.8
4区 生物学
Q4 BIOPHYSICS
引用次数: 0
摘要
脊椎动物杆状和锥状光感受器中的视网膜膜观酰基环化酶(RetGCs)被称为观酰基环化酶激活蛋白(GCAP1-7)的神经元Ca2+传感器蛋白家族激活。来自斑马鱼光感受器的GCAP5与RetGC结合,并赋予Ca2+/Fe2+依赖性的RetGC酶活性调节,促进视觉光传导的恢复阶段。我们报道了具有R22A突变(称为GCAP5R22A)的GCAP5的NMR化学位移定位,该突变可以消除蛋白质二聚化并激活RetGC,其活性比野生型GCAP5 (BMRB No. 51,783)高3倍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
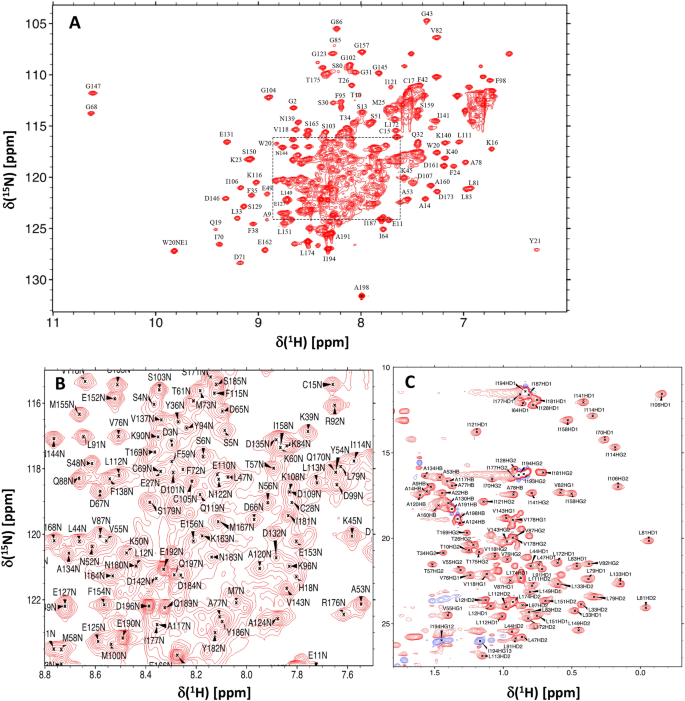
Chemical shift assignments of retinal guanylyl cyclase activating protein 5 (GCAP5) with a mutation (R22A) that abolishes dimerization and enhances cyclase activation
Retinal membrane guanylyl cyclases (RetGCs) in vertebrate rod and cone photoreceptors are activated by a family of neuronal Ca2+ sensor proteins called guanylyl cyclase activating proteins (GCAP1-7). GCAP5 from zebrafish photoreceptors binds to RetGC and confers Ca2+/Fe2+-dependent regulation of RetGC enzymatic activity that promotes the recovery phase of visual phototransduction. We report NMR chemical shift assignments of GCAP5 with a R22A mutation (called GCAP5R22A) that abolishes protein dimerization and activates RetGC with 3-fold higher activity than that of wild type GCAP5 (BMRB No. 51,783).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


