CEA和ca15 -3对乳腺癌患者化疗监测的诊断意义。
Q3 Medicine
Journal of Circulating Biomarkers
Pub Date : 2022-11-07
eCollection Date: 2022-01-01
DOI:10.33393/jcb.2022.2446
引用次数: 5
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。

Diagnostic impact of CEA and CA 15-3 on chemotherapy monitoring of breast cancer patients.
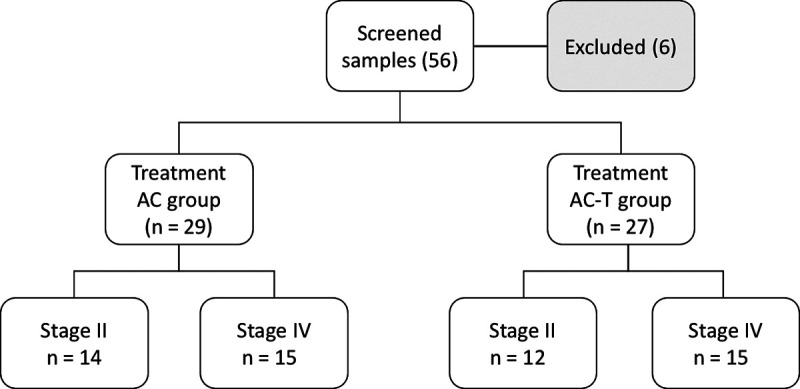
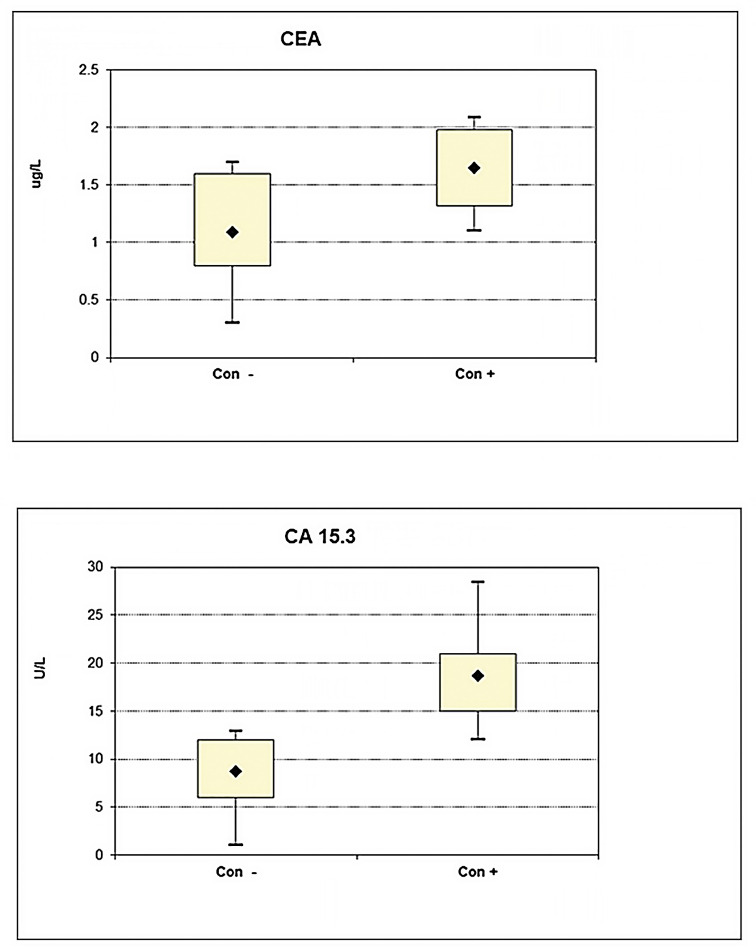
ABSTRACT Introduction: Serum tumor markers have emerged as an effective tool to determine prognosis and treatment efficiency in different cancer types. This study aimed to explore the chemotherapy monitoring efficiency and prognostic sensitivity of tumor-associated cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) in early (II) and late (IV) clinical stage breast cancer. Methods: CA 15-3 and CEA serum levels were assessed in 56 breast cancer patients at early (n = 26) and late (n = 30) clinical stages with these primary inclusion criteria: those who received adjuvant chemotherapy AC (adriamycin and cyclophosphamide) or AC-T (adriamycin and cyclophosphamide followed by taxane) regimens and possessed tumors negative for human epidermal growth factor receptor 2 (HER2) based on a particle-enhanced turbidimetric assay. Results: CA 15-3 had a higher elevation than CEA in the pretreatment group of breast cancer patients when compared to healthy controls. Late-stage patients showed higher positive serum levels than early-stage patients for both markers, with a preference for CA 15-3 over CEA. AC-T chemotherapy regimen treatment in both clinical stages revealed a significantly higher level of both markers as compared to the AC regime, with a preference for CA 15-3 over CEA in late stage. Both markers were significantly higher in the late-stage group as compared to early-stage groups for both chemotherapy regimens. Conclusions: CA 15-3 is more efficient as a prognostic monitoring marker than CEA and reveals a positive connection between chemotherapy regimen system and staging, with increased observability in late-stage patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Circulating Biomarkers
Medicine-Biochemistry (medical)
CiteScore
3.20
自引率
0.00%
发文量
9
审稿时长
8 weeks
期刊介绍:
Journal of Circulating Biomarkers is an international, peer-reviewed, open access scientific journal focusing on all aspects of the rapidly growing field of circulating blood-based biomarkers and diagnostics using circulating protein and lipid markers, circulating tumor cells (CTC), circulating cell-free DNA (cfDNA) and extracellular vesicles, including exosomes, microvesicles, microparticles, ectosomes and apoptotic bodies. The journal publishes high-impact articles that deal with all fields related to circulating biomarkers and diagnostics, ranging from basic science to translational and clinical applications. Papers from a wide variety of disciplines are welcome; interdisciplinary studies are especially suitable for this journal. Included within the scope are a broad array of specialties including (but not limited to) cancer, immunology, neurology, metabolic diseases, cardiovascular medicine, regenerative medicine, nosology, physiology, pathology, technological applications in diagnostics, therapeutics, vaccine, drug delivery, regenerative medicine, drug development and clinical trials. The journal also hosts reviews, perspectives and news on specific topics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


