对表达NISTmAb参比抗体Fab片段的酵母进行骨架核磁共振鉴定
IF 0.8
4区 生物学
Q4 BIOPHYSICS
引用次数: 2
摘要
单克隆抗体(mAb)蛋白类已成为生产新的救命药物产品的主要治疗平台。单克隆抗体由抗原结合片段(Fab)和可结晶片段(Fc)两个结构域组成。尽管在临床上取得了成功,但完整的Fab结构域的NMR分配一直难以实现,部分原因是由于正确折叠,三重标记的2H,13C,15N Fab结构域的生产存在问题。在这里,我们报道了在酵母中成功地重组表达了一个三重标记的Fab结构域,该结构域来自标准IgG1κ,称为NISTmAb。利用2H,13C,15N Fab结构域,我们分配了94%的1H, 13C和15N主链原子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
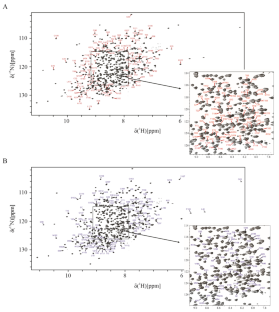
Backbone NMR assignment of the yeast expressed Fab fragment of the NISTmAb reference antibody
The monoclonal antibody (mAb) protein class has become a primary therapeutic platform for the production of new life saving drug products. MAbs are comprised of two domains: the antigen-binding fragment (Fab) and crystallizable fragment (Fc). Despite the success in the clinic, NMR assignments of the complete Fab domain have been elusive, in part due to problems in production of properly folded, triply-labeled 2H,13C,15N Fab domain. Here, we report the successful recombinant expression of a triply-labeled Fab domain, derived from the standard IgG1κ known as NISTmAb, in yeast. Using the 2H,13C,15N Fab domain, we assigned 94% of the 1H, 13C, and 15N backbone atoms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomolecular NMR Assignments
生物-光谱学
CiteScore
1.70
自引率
11.10%
发文量
59
审稿时长
6-12 weeks
期刊介绍:
Biomolecular NMR Assignments provides a forum for publishing sequence-specific resonance assignments for proteins and nucleic acids as Assignment Notes. Chemical shifts for NMR-active nuclei in macromolecules contain detailed information on molecular conformation and properties.
Publication of resonance assignments in Biomolecular NMR Assignments ensures that these data are deposited into a public database at BioMagResBank (BMRB; http://www.bmrb.wisc.edu/), where they are available to other researchers. Coverage includes proteins and nucleic acids; Assignment Notes are processed for rapid online publication and are published in biannual online editions in June and December.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


