重新考虑和修订了蛇气孔门的属界。
摘要
Ophiostomatales建于1980年。从那时起,其中几个属被重新定义,其他属也被描述。目前该目共有14个可接受属。它们包括植物和人类疾病的病原体,以及树皮甲虫等昆虫的常见伙伴。众所周知的例子包括荷兰榆树病真菌和人类和动物孢子丝菌病的病原体。多年来,Opiostotales的分类学一直很混乱,主要是由于用于划分不相关真菌分类群的形态特征的趋同进化。基于DNA的方法的出现在很大程度上解决了这种困惑。然而,一些属的划分以及各种物种和较小谱系的分布仍然没有定论。在这项研究中,我们重新考虑了Ophiostomales的属界。根据代表该目主要属的31个物种的全基因组序列数据构建的系统发育学框架被用作划分属的指南。该框架还为我们从目前最常用的基因区域中选择最佳标记以进行这些真菌的分类学研究提供了依据。对200多个物种的DNA进行了扩增和测序,代表了该目的所有谱系。我们基于不同的基因区域构建了系统发育树,并利用一系列系统发育分析组装了一个串联的数据集。结果支持并证实了目前接受的14个属中的9个属的划分,即Aureovirgo、Ceratocystiopsis、Esteya、Fragosphaeria、Graphilbum、Hawksworthiomyces、Ophiostoma、Raffaelea和Sporothrix。最近描述的两个属,Chrysosphaeria和Intubia,没有包括在多基因座分析中。这是由于它们的高度序列差异,这被证明导致了不明确的分类位置,尽管系统发育学分析结果支持它们被纳入Ophiostomatales。除了目前被接受的Ophiostomales属之外,还出现了与这些属不同的、得到充分支持的谱系。这些被描述为新属。两个谱系包括Grosmania和Dryadomyces的模式种,因此恢复了这些属,并重新定义了它们的范围。在需要的地方,对Ophiostomales中所有属的描述进行了标准化和精细化,为新出现的属中的物种提供了39个新组合,为孢子丝提供了一个新组合。根据现有数据,无法确认Afrorafflea的位置,该属已被视为Ophiostomales中的自始景天属(incertae sedis)。由于缺乏可用于该单型化石属的活材料,古安布罗西亚未被纳入本研究。总的来说,这项研究提供了目前可能的最全面、最稳健的Opiotomata目系统发育。它还澄清了与该目相关的几个尚未解决的“一真菌一名称”命名问题。分类新颖:新属:Harringtonia Z.W.de Beer&M.Procter,Heinzbutinia Z.W de Beer和M.Procter、Jamesreidia Z.W.de Beer&M.Procter、Masuyamyces Z.W。新种:Masuyamyces massonianae M.Procter&Z.W.de Beer。新组合:蒙特氏干酵母(M.Morelet)M.Procter&Z.W.de Beer、quercivorus干酵母(Kubono&Shin.Ito)M.Procter&Z.W.der Beer、蒙古干酵母(K.H.Kim等人)M.Proctor&Z.W.Deer、硫生干酵母(L.R.Batra)M.Procters&Z.W.de Beer、Graphilbum pusillum(Masuya)M.Proctor&Z.W.de Beer、黄地黄(K.Jacobs&M.J.Wingf.)M。Procter&Z.W.de Beer,Grossmania altior,Grossmania gestamen(de Errasti&Z.W.de Beer)M.Procter&Z.W.de Beer,Grossmannia innermongolica(X.W.Liu et al.)M.Proctor&Z.W.de Beer,grossmania pistaciae(Paciura et al.)M.Procter&Z.W.de Beer,Gross mania pruni(Masuya&M.J.Wingf。Procter&Z.W.de Beer,Harringtonia aguacate,Heinzbutinia solheimii(B.Strzałka&Jankowiak)Z.W.de Beer&M.Procter,James coronata(Olchow.&J.Reid)M.Procter&Z.W.der Beer,James nigricarpa(R.W。 Davidson)M.Procter&Z.W.de Beer,Jamesreidia rostrocoronata,拟铕细颗粒菌(Olchow.&J.Reid)M.Procter&Z.W.de Beer,辐射细颗粒菌。Procter&Z.W.de Beer,Masuyamyces lotiformis。引文:de Beer W,Procter M,Wingfield MJ,Marincowitz S,Duong TA(2022)。重新考虑并修订了Opiostotales的属界。真菌学研究101:57-120。doi:10.3114/sim.2022.101.02。
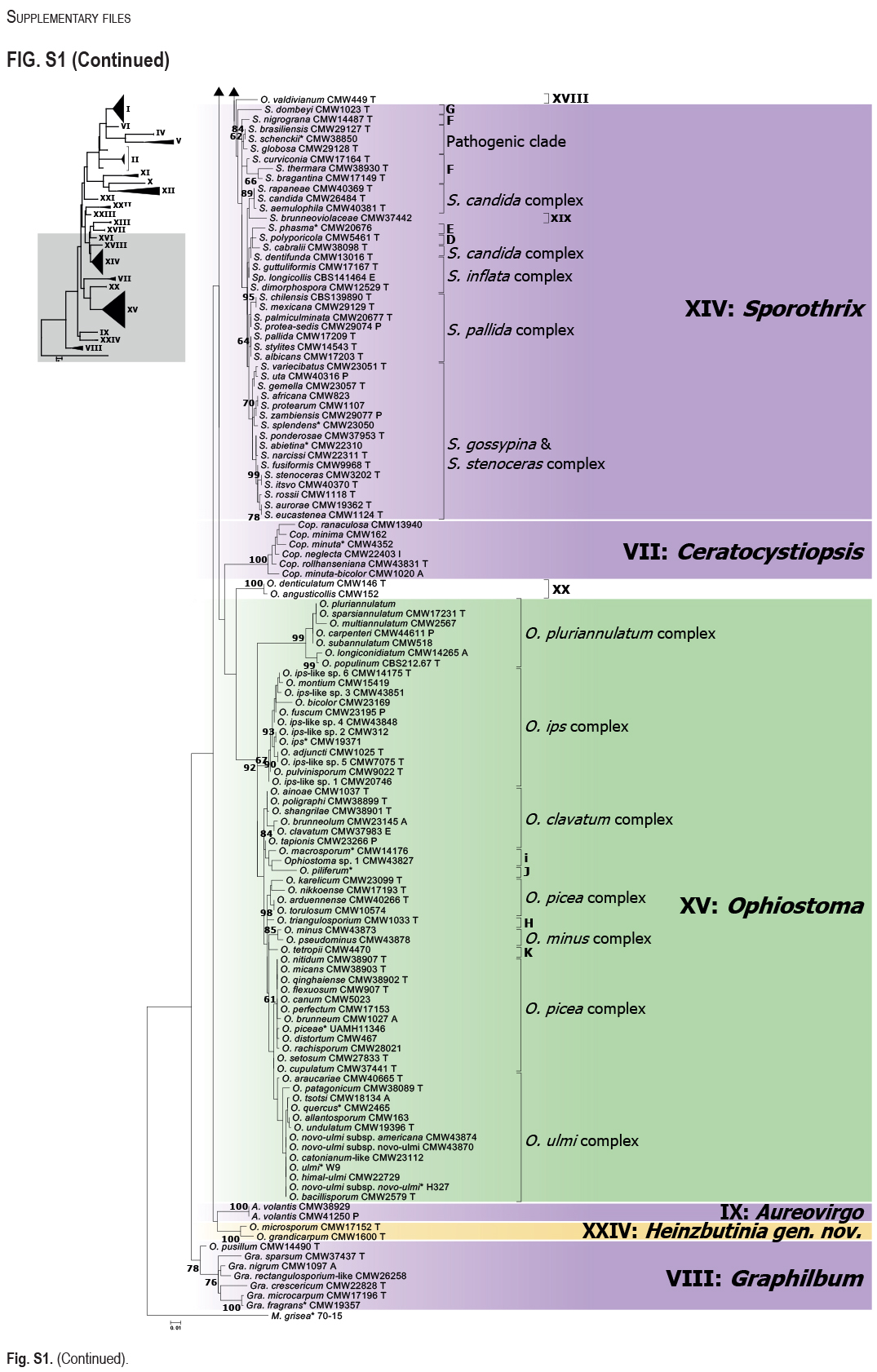
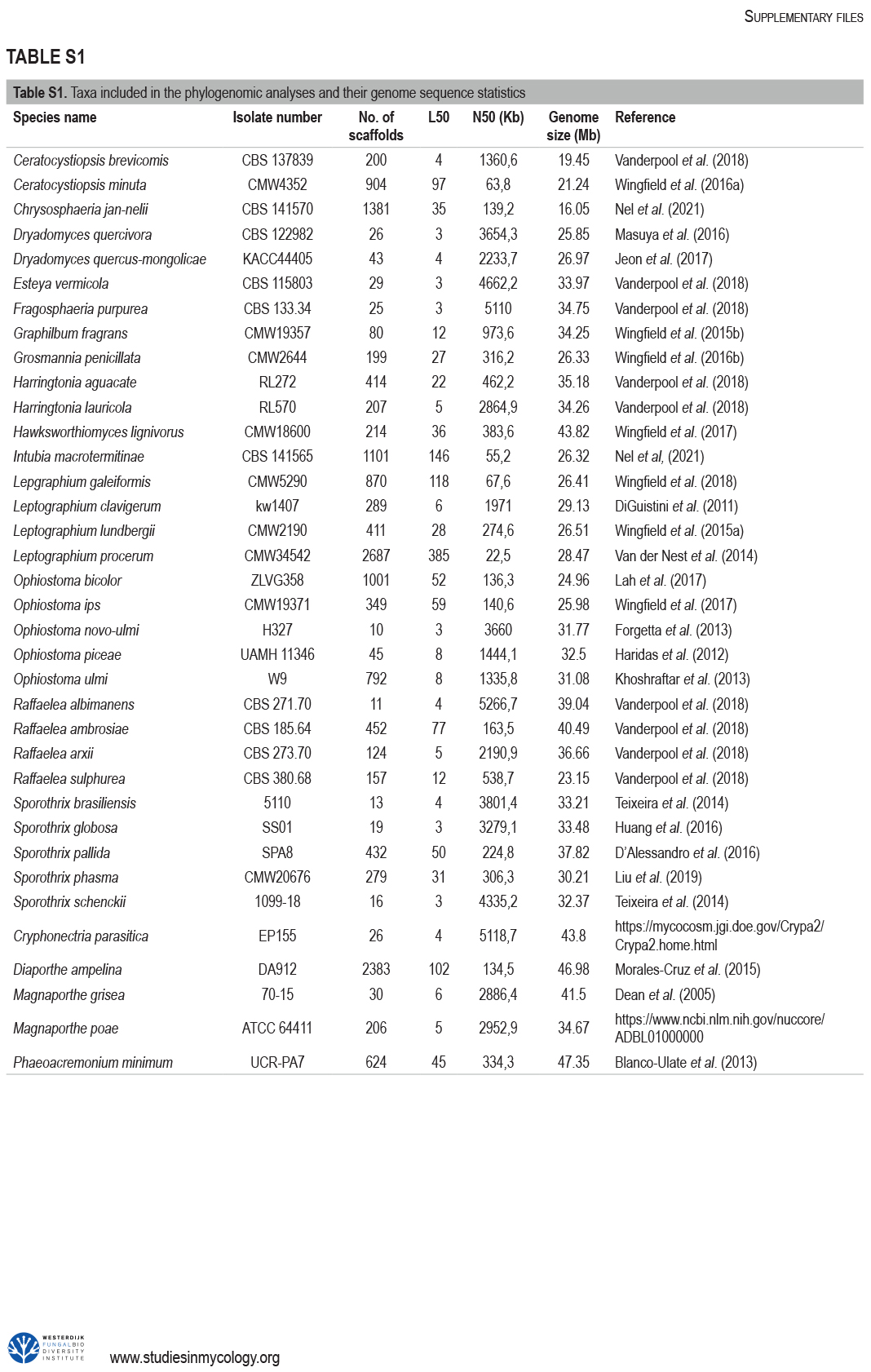
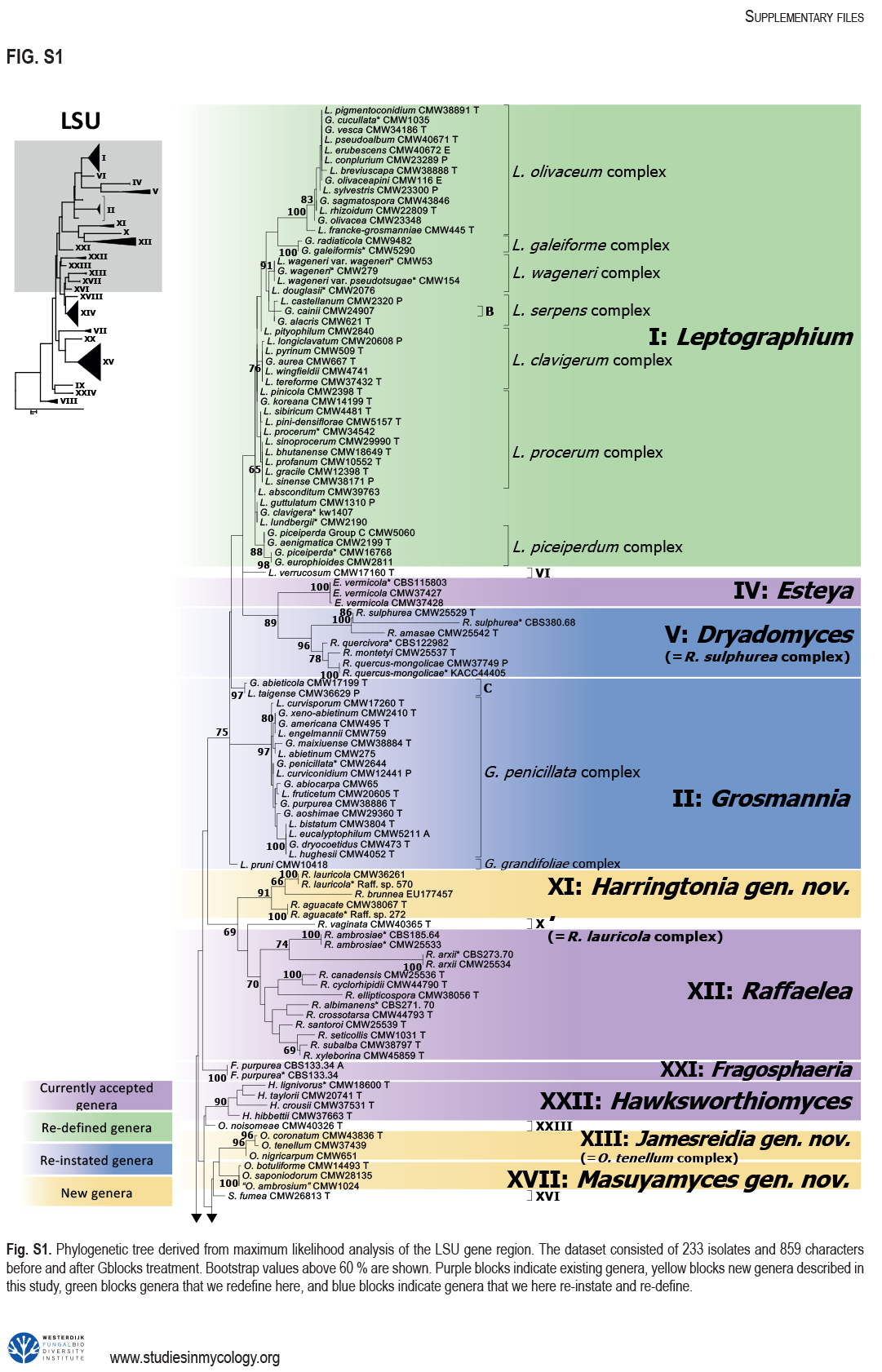
The Ophiostomatales was erected in 1980. Since that time, several of the genera have been redefined and others have been described. There are currently 14 accepted genera in the Order. They include species that are the causal agents of plant and human diseases and common associates of insects such as bark beetles. Well known examples include the Dutch elm disease fungi and the causal agents of sporotrichosis in humans and animals. The taxonomy of the Ophiostomatales was confused for many years, mainly due to the convergent evolution of morphological characters used to delimit unrelated fungal taxa. The emergence of DNA-based methods has resolved much of this confusion. However, the delineation of some genera and the placement of various species and smaller lineages remains inconclusive. In this study we reconsidered the generic boundaries within the Ophiostomatales. A phylogenomic framework constructed from genome-wide sequence data for 31 species representing the major genera in the Order was used as a guide to delineate genera. This framework also informed our choice of the best markers from the currently most commonly used gene regions for taxonomic studies of these fungi. DNA was amplified and sequenced for more than 200 species, representing all lineages in the Order. We constructed phylogenetic trees based on the different gene regions and assembled a concatenated data set utilising a suite of phylogenetic analyses. The results supported and confirmed the delineation of nine of the 14 currently accepted genera, i.e. Aureovirgo, Ceratocystiopsis, Esteya, Fragosphaeria, Graphilbum, Hawksworthiomyces, Ophiostoma, Raffaelea and Sporothrix. The two most recently described genera, Chrysosphaeria and Intubia, were not included in the multi-locus analyses. This was due to their high sequence divergence, which was shown to result in ambiguous taxonomic placement, even though the results of phylogenomic analysis supported their inclusion in the Ophiostomatales. In addition to the currently accepted genera in the Ophiostomatales, well-supported lineages emerged that were distinct from those genera. These are described as novel genera. Two lineages included the type species of Grosmannia and Dryadomyces and these genera are thus reinstated and their circumscriptions redefined. The descriptions of all genera in the Ophiostomatales were standardised and refined where this was required and 39 new combinations have been provided for species in the newly emerging genera and one new combination has been provided for Sporothrix. The placement of Afroraffaelea could not be confirmed using the available data and the genus has been treated as incertae sedis in the Ophiostomatales. Paleoambrosia was not included in this study, due to the absence of living material available for this monotypic fossil genus. Overall, this study has provided the most comprehensive and robust phylogenies currently possible for the Ophiostomatales. It has also clarified several unresolved One Fungus-One Name nomenclatural issues relevant to the Order. Taxonomic novelties: New genera: Harringtonia Z.W. de Beer & M. Procter, Heinzbutinia Z.W. de Beer & M. Procter, Jamesreidia Z.W. de Beer & M. Procter, Masuyamyces Z.W. de Beer & M. Procter. New species: Masuyamyces massonianae M. Procter & Z.W. de Beer. New combinations: Dryadomyces montetyi (M. Morelet) M. Procter & Z.W. de Beer, Dryadomyces quercivorus (Kubono & Shin. Ito) M. Procter & Z.W. de Beer, Dryadomyces quercus-mongolicae (K.H. Kim et al.) M. Procter & Z.W. de Beer, Dryadomyces sulphureus (L.R. Batra) M. Procter & Z.W. de Beer, Graphilbum pusillum (Masuya) M. Procter & Z.W. de Beer, Grosmannia abieticolens (K. Jacobs & M.J. Wingf.) M. Procter & Z.W. de Beer, Grosmannia altior (Paciura et al.) M. Procter & Z.W. de Beer, Grosmannia betulae (Jankowiak et al.) M. Procter & Z.W. de Beer, Grosmannia curviconidia (Paciura et al.) M. Procter & Z.W. de Beer, Grosmannia euphyes (K. Jacobs & M.J. Wingf.) M. Procter & Z.W. de Beer, Grosmannia fenglinhensis (R. Chang et al.) M. Procter & Z.W. de Beer, Grosmannia gestamen (de Errasti & Z.W. de Beer) M. Procter & Z.W. de Beer, Grosmannia innermongolica (X.W. Liu et al.) M. Procter & Z.W. de Beer, Grosmannia pistaciae (Paciura et al.) M. Procter & Z.W. de Beer, Grosmannia pruni (Masuya & M.J. Wingf.) M. Procter & Z.W. de Beer, Grosmannia taigensis (Linnak. et al.) M. Procter & Z.W. de Beer, Grosmannia trypodendri (Jankowiak et al.) M. Procter & Z.W. de Beer, Harringtonia aguacate (D.R. Simmons et al.) M. Procter & Z.W. de Beer, Harringtonia brunnea (L.R. Batra) M. Procter & Z.W. de Beer, Harringtonia lauricola (T.C. Harr. et al.) Z.W. de Beer & M. Procter, Heinzbutinia grandicarpa (Kowalski & Butin) Z.W. de Beer & M. Procter, Heinzbutinia microspora (Arx) M. Procter & Z.W. de Beer, Heinzbutinia solheimii (B. Strzałka & Jankowiak) Z.W. de Beer & M. Procter, Jamesreidia coronata (Olchow. & J. Reid) M. Procter & Z.W. de Beer, Jamesreidia nigricarpa (R.W. Davidson) M. Procter & Z.W. de Beer, Jamesreidia rostrocoronata (R.W. Davidson & Eslyn) M. Procter & Z.W. de Beer, Jamesreidia tenella (R.W. Davidson) Z.W. de Beer & M. Procter, Leptographium cainii (Olchow. & J. Reid) M. Procter & Z.W. de Beer, Leptographium europioides (E.F. Wright & Cain) M. Procter & Z.W. de Beer, Leptographium galeiforme (B.K. Bakshi) M. Procter & Z.W. de Beer, Leptographium pseudoeurophioides (Olchow. & J. Reid) M. Procter & Z.W. de Beer, Leptographium radiaticola (J.J. Kim et al.) M. Procter & Z.W. de Beer, Masuyamyces acarorum (R. Chang & Z.W. de Beer) M. Procter & Z.W. de Beer, Masuyamyces ambrosius (B.K. Bakshi) M. Procter & Z.W. de Beer, Masuyamyces botuliformis (Masuya) Z.W. de Beer & M. Procter, Masuyamyces jilinensis (R. Chang et al.) M. Procter & Z.W. de Beer, Masuyamyces lotiformis (Z. Wang & Q. Lu) M. Procter & Z.W. de Beer, Masuyamyces pallidulus (Linnak. et al.) M. Procter & Z.W. de Beer, Masuyamyces saponiodorus (Linnak. et al.) M. Procter & Z.W. de Beer, Sporothrix longicollis (Massee & E.S. Salmon) M. Procter & Z.W. de Beer. Citation: de Beer W, Procter M, Wingfield MJ, Marincowitz S, Duong TA (2022). Generic boundaries in the Ophiostomatales reconsidered and revised. Studies in Mycology 101: 57-120. doi: 10.3114/sim.2022.101.02.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


