通过受控开环偏聚共聚合成可降解聚合物的多功能方法
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 38
摘要
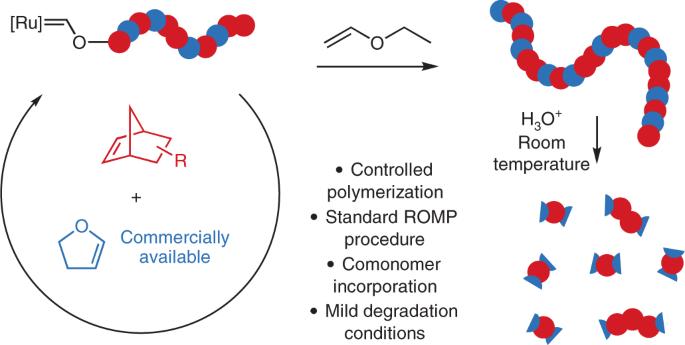
降冰片烯衍生物(NBE)是活环开环偏聚聚合反应的常见单体,可生成分散性低、功能多样的聚合物。然而,聚降冰片烯的全碳骨架是不可降解的。在此,我们报告了一种通过 2,3-二氢呋喃与 NBEs 共聚合合成可降解聚合物的方法。2,3-二氢呋喃与 Grubbs 催化剂迅速反应,形成热力学稳定的 Ru Fischer 碳烯--共聚过程中唯一可检测到的活性 Ru 物种--而 NBE 的加入则决定了反应的速率。这种反应性减弱了 NBE 的均加性,使可酸性降解的烯醇醚链节均匀地加入到整个共聚物中,从而使聚合物完全降解,同时保持活环开环偏聚聚合的有利特性。2,3-二氢呋喃与 NBE 的共聚产生了低分散性聚合物,其溶解度、玻璃化温度和机械性能均可调。在弱酸性条件下,这些聚合物可完全降解为小分子或低聚物。这种方法可以很容易地应用于广泛使用的 NBE 的传统开环偏聚聚合反应,从而合成出性能可调的易降解聚合物,用于各种应用和环境可持续发展。可降解聚合物对于技术应用和可持续发展非常重要,但它们仍然难以通过开环元合成聚合反应(ROMP)获得。现在,商用 2,3-二氢呋喃被证明是各种降冰片烯的有效 ROMP 共聚单体。这种共聚物可生成具有可控分子量、不同官能度和可调特性的新型酸降解聚合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A versatile approach for the synthesis of degradable polymers via controlled ring-opening metathesis copolymerization
Norbornene derivatives (NBEs) are common monomers for living ring-opening metathesis polymerization and yield polymers with low dispersities and diverse functionalities. However, the all-carbon backbone of poly-NBEs is non-degradable. Here we report a method to synthesize degradable polymers by copolymerizing 2,3-dihydrofuran with NBEs. 2,3-Dihydrofuran rapidly reacts with Grubbs catalyst to form a thermodynamically stable Ru Fischer carbene—the only detectable active Ru species during copolymerization—and the addition of NBEs becomes rate determining. This reactivity attenuates the NBE homoaddition and allows uniform incorporation of acid-degradable enol ether linkages throughout the copolymers, which enables complete polymer degradation while maintaining the favourable characteristics of living ring-opening metathesis polymerization. Copolymerization of 2,3-dihydrofuran with NBEs gives low dispersity polymers with tunable solubility, glass transition temperature and mechanical properties. These polymers can be fully degraded into small molecule or oligomeric species under mildly acidic conditions. This method can be readily adapted to traditional ring-opening metathesis polymerization of widely used NBEs to synthesize easily degradable polymers with tunable properties for various applications and for environmental sustainability. Degradable polymers are important for technological applications and sustainability, but they remain difficult to access via ring-opening metathesis polymerization (ROMP). Now, commercial 2,3-dihydrofuran is shown to be an effective ROMP comonomer for various norbornenes. This copolymerization generates new acid-degradable polymers with controlled molecular weights, different functionalities and tunable properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


