SLC25A39 是哺乳动物细胞线粒体谷胱甘肽输入的必要条件
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 57
摘要
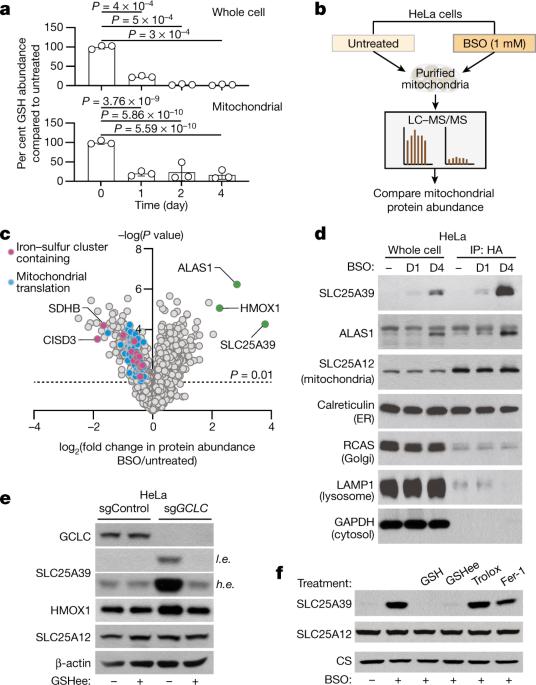
谷胱甘肽(GSH)是一种小分子硫醇,在所有真核生物中含量丰富,在氧化代谢中起着关键作用1。线粒体作为氧化反应的主要场所,必须保持足够的 GSH 水平,以发挥保护和生物合成功能2。GSH 完全在细胞质中合成,但线粒体 GSH 输入的分子机制仍不清楚。在这里,我们利用细胞器蛋白质组学和代谢组学方法,确定了 SLC25A39(一种功能未知的线粒体膜载体)是 GSH 向线粒体转运的调控因子。SLC25A39 的缺失会减少线粒体 GSH 的输入和丰度,但不会影响细胞 GSH 水平。同时缺乏 SLC25A39 及其同源物 SLC25A40 的细胞在含有铁硫簇的蛋白质的活性和稳定性方面表现出缺陷。我们发现,线粒体 GSH 导入是体外细胞增殖和小鼠红细胞发育所必需的。在线粒体中异源表达一种工程化的双功能细菌 GSH 生物合成酶(GshF)可使线粒体产生 GSH,并改善因其耗竭而导致的代谢和增殖缺陷。最后,GSH 的可用性负向调节 SLC25A39 蛋白的丰度,将哺乳动物细胞中的氧化还原平衡与线粒体 GSH 输入联系起来。我们的研究发现,SLC25A39是线粒体GSH导入机制中一个重要的调节成分。SLC25A39 及其同源物 SLC25A40 在将谷胱甘肽导入哺乳动物细胞线粒体的过程中具有冗余作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells
Glutathione (GSH) is a small-molecule thiol that is abundant in all eukaryotes and has key roles in oxidative metabolism1. Mitochondria, as the major site of oxidative reactions, must maintain sufficient levels of GSH to perform protective and biosynthetic functions2. GSH is synthesized exclusively in the cytosol, yet the molecular machinery involved in mitochondrial GSH import remains unknown. Here, using organellar proteomics and metabolomics approaches, we identify SLC25A39, a mitochondrial membrane carrier of unknown function, as a regulator of GSH transport into mitochondria. Loss of SLC25A39 reduces mitochondrial GSH import and abundance without affecting cellular GSH levels. Cells lacking both SLC25A39 and its paralogue SLC25A40 exhibit defects in the activity and stability of proteins containing iron–sulfur clusters. We find that mitochondrial GSH import is necessary for cell proliferation in vitro and red blood cell development in mice. Heterologous expression of an engineered bifunctional bacterial GSH biosynthetic enzyme (GshF) in mitochondria enables mitochondrial GSH production and ameliorates the metabolic and proliferative defects caused by its depletion. Finally, GSH availability negatively regulates SLC25A39 protein abundance, coupling redox homeostasis to mitochondrial GSH import in mammalian cells. Our work identifies SLC25A39 as an essential and regulated component of the mitochondrial GSH-import machinery. SLC25A39 and its paralogue SLC25A40 have redundant roles in the import of glutathione into mitochondria of mammalian cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


