尿Etiocholanolone/Androsterone比值替代血清睾酮/双氢睾酮比值诊断印度尼西亚5例α -还原酶2型缺乏患者和携带者的准确性
IF 1.8
Q3 ENDOCRINOLOGY & METABOLISM
International Journal of Endocrinology and Metabolism
Pub Date : 2021-04-18
eCollection Date: 2021-04-01
DOI:10.5812/ijem.109510
引用次数: 1
摘要
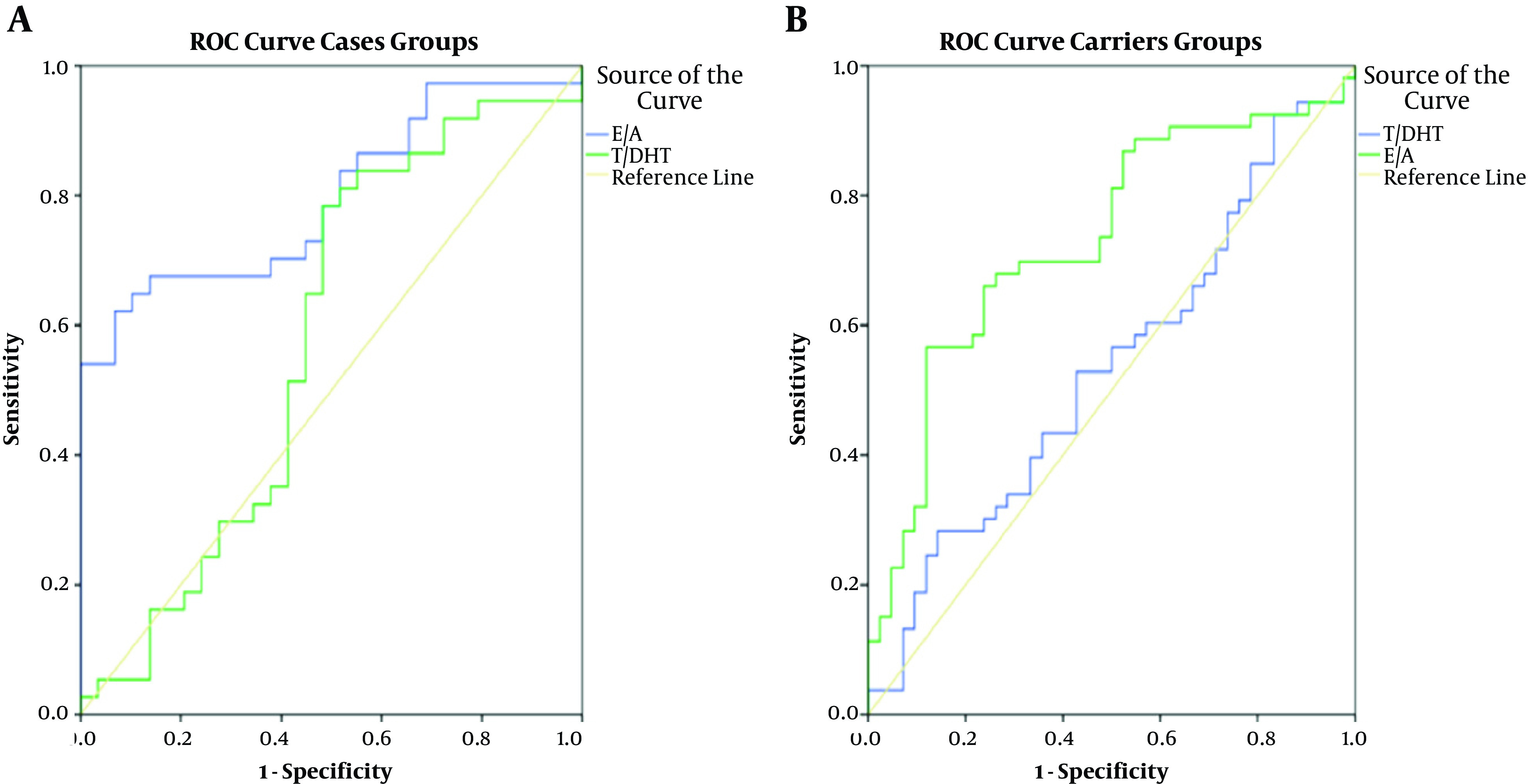
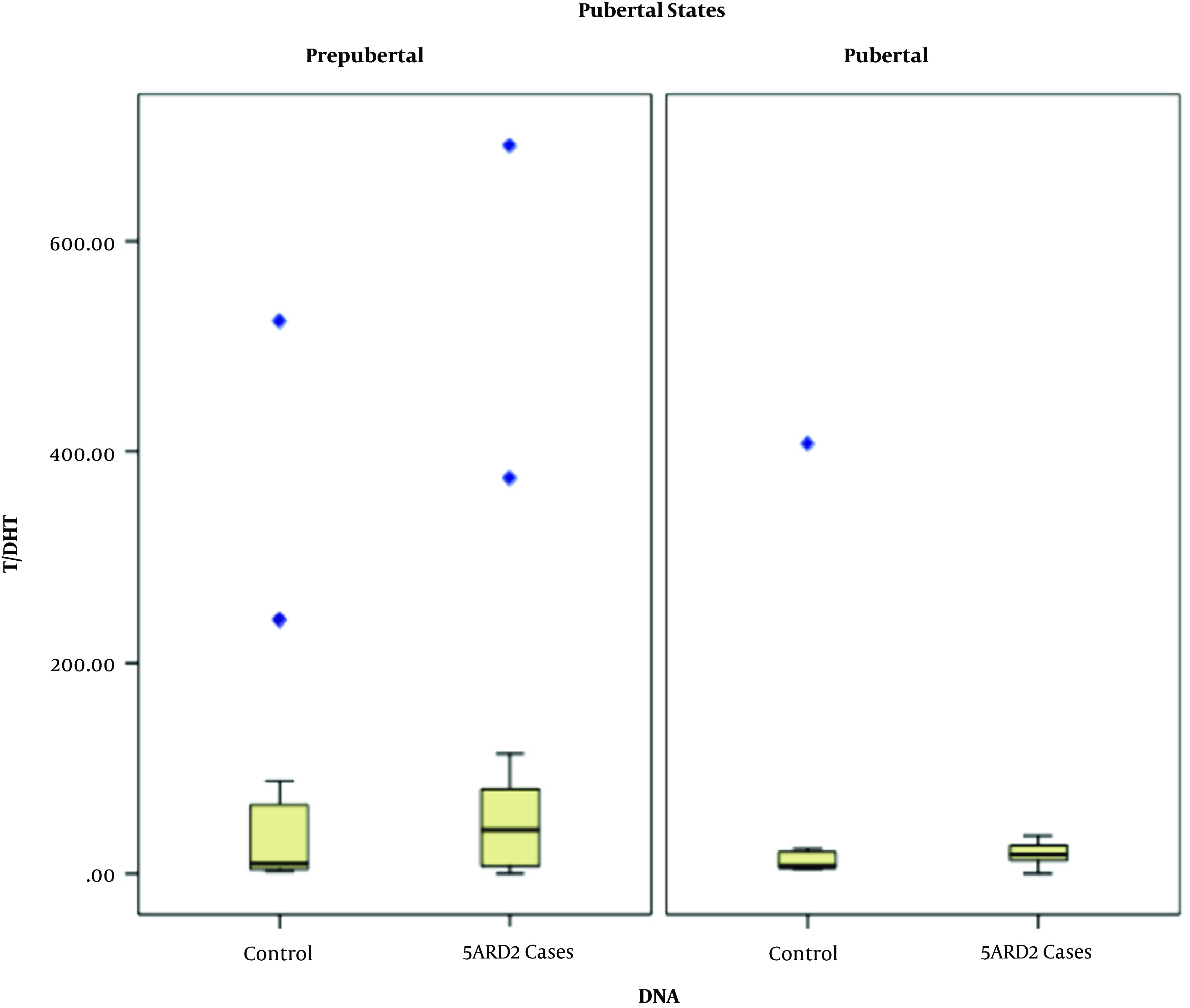
背景:5α -还原酶2型缺乏症(5ARD2)是一种遗传性疾病,临床表现为46,xy患者不同程度的低男性化。在5ARD2的诊断途径中,在对SRD5A2基因进行分子分析之前,广泛采用睾酮/双氢睾酮(T/DHT)比值。然而,由于成本效益的考虑,DHT检测在我国通常缺乏临床设置;因此,我们考虑采用尿etiocholanolone/androsterone (Et/An)比值作为替代测试。目的:我们旨在确定尿Et/An比与T/DHT比在诊断5ARD2患者和携带者中的诊断价值。方法:招募66例疑似5ard46、XY性发育障碍(DSD)患者及95名家庭成员。分析患者的临床表现、T/DHT、尿Et/An比值及SRD5A2基因。以SRD5A2基因分子分析为金标准,采用受试者工作特征(ROC)曲线分析比较两种比值诊断5ARD2患者和携带者的准确性。结果:分子确诊5ARD2患者37例,其余29例为正常对照组,携带者组53例为携带者,对照组42例。诊断5ARD2患者T/DHT和尿Et/An比值的auc(曲线下面积)分别为57.7% (95% CI 43.0 ~ 72.4%, P > 0.05)和79.7% (95% CI 69.0 ~ 90.4%, P < 0.001),诊断携带者分别为54.1% (95% CI 42.4 ~ 65.8%, P > 0.05)和75.1% (95% CI 65.1 ~ 85.1%, P < 0.001)。5ARD2患者尿Et/An比值临界值≥0.95,携带者尿Et/An比值临界值≥0.99。结论:睾酮/DHT比值在诊断5ARD2患者时是不准确的。当缺乏SRD5A2基因的分子分析时,尿Et/An比值可能是诊断5ARD2患者和携带者的有用测试。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Accuracy of Urinary Etiocholanolone/Androsterone Ratio as Alternative to Serum Testosterone/Dihydrotestosterone Ratio for Diagnosis of 5 Alpha-reductase Type 2 Deficiency Patients and Carriers in Indonesia.
Background The 5 Alpha-reductase type 2 deficiency (5ARD2) is an inherited condition, which clinically presents as variable degrees of under virilization in affected 46,XY individuals. In the diagnostic pathway of 5ARD2, the testosterone/dihydrotestosterone (T/DHT) ratio is broadly employed before molecular analysis of the SRD5A2 gene. However, due to cost-benefit considerations, the DHT test in our country is routinely lacking in clinical settings; therefore, we considered applying the urinary etiocholanolone/androsterone (Et/An) ratio as an alternative test. Objectives We aimed to determine the diagnostic value of the urinary Et/An ratio versus the T/DHT ratio in diagnosing 5ARD2 patients and carriers. Methods Sixty-six suspected 5ARD2 46,XY disorders of sex development (DSD) individuals and 95 family members were recruited in the study. Their clinical manifestations, T/DHT and urinary Et/An ratios, and SRD5A2 genes were analyzed. Using molecular analysis of the SRD5A2 gene as the gold standard, we compared the accuracy of both ratios in diagnosing 5ARD2 patients and carriers with receiver operating characteristic (ROC) curve analysis. Results Thirty-seven patients were confirmed molecularly to have 5ARD2, and the rest (n = 29) were assessed as normal controls, while in the carrier group, 53 were molecularly confirmed as carriers and 42 as controls. The AUCs (areas under the curve) of the T/DHT and urinary Et/An ratios were 57.7% (95% CI 43.0 - 72.4%, P > 0.05) and 79.7% (95% CI 69.0 - 90.4%, P < 0.001), respectively, in diagnosing 5ARD2 patients and 54.1% (95% CI 42.4 - 65.8%, P > 0.05) and 75.1% (95% CI 65.1 - 85.1%, P < 0.001), respectively, in diagnosing carriers. The cutoff value of the urinary Et/An ratio was set at ≥ 0.95 for detecting 5ARD2 patients and ≥ 0.99 for detecting carriers. Conclusions The testosterone/DHT ratio was inaccurate in diagnosing 5ARD2 patients. When molecular analysis for the SRD5A2 gene is lacking, the urinary Et/An ratio may be a useful test to diagnose 5ARD2 patients and carriers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Endocrinology and Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
3.10
自引率
4.80%
发文量
0
期刊介绍:
The aim of the International Journal of Endocrinology and Metabolism (IJEM) is to increase knowledge, stimulate research in the field of endocrinology, and promote better management of patients with endocrinological disorders. To achieve this goal, the journal publishes original research papers on human, animal and cell culture studies relevant to endocrinology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


