幽门螺杆菌IV型分泌系统通过CagA-和jnk依赖性途径上调上皮内皮质蛋白的表达
IF 1.6
2区 生物学
Q3 CELL BIOLOGY
引用次数: 8
摘要
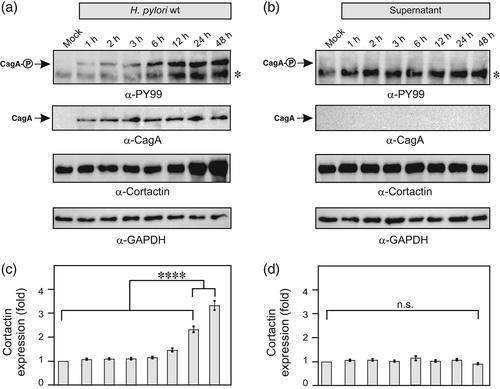
皮质蛋白是一种重要的肌动蛋白结合因子,它控制着宿主细胞中肌动蛋白-细胞骨架的重塑。通过这种方式,接触已被证明在细胞运动和肿瘤细胞侵袭中都表现出重要的功能。此外,接触蛋白基因cttn在各种癌症类型的人类中被扩增。幽门螺杆菌是多种胃疾病的病原体,是胃腺癌发生的重要危险因素。多次研究表明,幽门螺杆菌操纵感染的胃上皮细胞中与癌症相关的信号转导事件,如接触蛋白的磷酸化状态。事实上,幽门螺杆菌通过改变接触蛋白结合伙伴的活性来刺激肌动蛋白-细胞骨架、细胞粘附和运动性的变化。本研究表明,幽门螺杆菌感染培养的AGS和Caco-2细胞24-48小时可导致蛋白水平上2-3倍的过表达。我们证明这种活性需要由cag致病性岛(cagPAI)和易位效应蛋白CagA编码的IV型分泌系统(T4SS)的完整性。我们进一步表明,CagA的异位表达足以刺激接触过表达。此外,在EPIYA-repeat区域的CagA磷酸化是不需要的,这表明这种CagA活性以磷酸化不依赖的方式进行。抑制剂研究进一步表明,参与的信号通路包括丝裂原活化蛋白激酶JNK (c-Jun n-末端激酶),而不是ERK1/2或p38。综上所述,利用幽门螺旋杆菌作为模型系统,本研究发现了一种以前未被识别的微生物病原体的接触蛋白激活级联。我们认为幽门螺杆菌靶向接触控制细胞结构和上皮屏障功能,从而影响胃癌的发展。结论幽门螺杆菌感染在蛋白水平诱导过表达cortnn,上调cortnn需要T4SS和效应蛋白CagA, CagA的异位表达足以刺激过表达cortnn,过表达cortnn引起CagA参与的宿主细胞信号通路包括MAP激酶JNK本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Helicobacter pylori type IV secretion system upregulates epithelial cortactin expression by a CagA- and JNK-dependent pathway
Cortactin represents an important actin‐binding factor, which controls actin‐cytoskeletal remodelling in host cells. In this way, cortactin has been shown to exhibit crucial functions both for cell movement and tumour cell invasion. In addition, the cortactin gene cttn is amplified in various cancer types of humans. Helicobacter pylori is the causative agent of multiple gastric diseases and represents a significant risk factor for the development of gastric adenocarcinoma. It has been repeatedly shown that H. pylori manipulates cancer‐related signal transduction events in infected gastric epithelial cells such as the phosphorylation status of cortactin. In fact, H. pylori modifies the activity of cortactin's binding partners to stimulate changes in the actin‐cytoskeleton, cell adhesion and motility. Here we show that H. pylori infection of cultured AGS and Caco‐2 cells for 24–48 hr leads to the overexpression of cortactin by 2–3 fold at the protein level. We demonstrate that this activity requires the integrity of the type IV secretion system (T4SS) encoded by the cag pathogenicity island (cagPAI) as well as the translocated effector protein CagA. We further show that ectopic expression of CagA is sufficient to stimulate cortactin overexpression. Furthermore, phosphorylation of CagA at the EPIYA‐repeat region is not required, suggesting that this CagA activity proceeds in a phosphorylation‐independent fashion. Inhibitor studies further demonstrate that the involved signalling pathway comprises the mitogen‐activated protein kinase JNK (c‐Jun N‐terminal kinase), but not ERK1/2 or p38. Taken together, using H. pylori as a model system, this study discovered a previously unrecognised cortactin activation cascade by a microbial pathogen. We suggest that H. pylori targets cortactin to manipulate the cellular architecture and epithelial barrier functions that can impact gastric cancer development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular Microbiology
生物-微生物学
CiteScore
9.70
自引率
0.00%
发文量
26
审稿时长
3 months
期刊介绍:
Cellular Microbiology aims to publish outstanding contributions to the understanding of interactions between microbes, prokaryotes and eukaryotes, and their host in the context of pathogenic or mutualistic relationships, including co-infections and microbiota. We welcome studies on single cells, animals and plants, and encourage the use of model hosts and organoid cultures. Submission on cell and molecular biological aspects of microbes, such as their intracellular organization or the establishment and maintenance of their architecture in relation to virulence and pathogenicity are also encouraged. Contributions must provide mechanistic insights supported by quantitative data obtained through imaging, cellular, biochemical, structural or genetic approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


