通过磺酰基引发的加成/6-外显环化,1,7-炔的新级联卤化成3,4-二氢喹啉-2(1H)- 1
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 86
摘要
以易于获得的芳基磺酰肼和NIS(或NBS)为原料,建立了一种新的1,7-炔级联三组分卤代磺化反应,可高效合成密集功能化的3,4-二氢喹啉-2(1H)- 1。该反应途径包括情景生成的磺酰基自由基触发的α、β共轭加成/6-外显式环化/自由基偶联序列,导致C-S、C-C和C-I(或C-Br)键连续多个成键事件,快速建立分子复杂性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
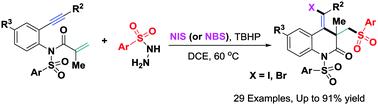
A new cascade halosulfonylation of 1,7-enynes toward 3,4-dihydroquinolin-2(1H)-ones via sulfonyl radical-triggered addition/6-exo-dig cyclization†
A new cascade three-component halosulfonylation of 1,7-enynes for efficient synthesis of densely functionalized 3,4-dihydroquinolin-2(1H)-ones has been established from readily accessible arylsulfonyl hydrazides and NIS (or NBS). The reaction pathway involves in situ-generated sulfonyl radical-triggered α,β-conjugated addition/6-exo-dig cyclization/radical coupling sequence, resulting in continuous multiple bond-forming events including C–S, C–C and C–I (or C–Br) bonds to rapidly build up molecular complexity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


