肉霉素。生物合成、合成和活性
IF 10.6
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 16
摘要
bottromycin是由多种链霉菌产生的一类大环肽天然产物,对临床相关的多药耐药病原体具有良好的抗菌活性。它们属于核糖体合成和翻译后修饰肽(RiPP)超家族的天然产物。该结构包含一个独特的四氨基酸大环,由一个罕见的氨基键、c -甲基化和一个d-氨基酸形成。本文综述了近年来(2009-2020年)博特霉素研究的各个方面,其中在全合成和对博特霉素生物合成的理解方面取得了重大进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
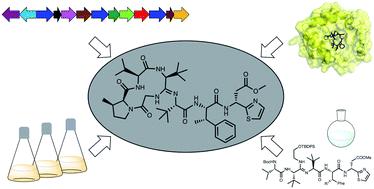
Bottromycins - biosynthesis, synthesis and activity
Covering: 1950s up to the end of 2020
Bottromycins are a class of macrocyclic peptide natural products that are produced by several Streptomyces species and possess promising antibacterial activity against clinically relevant multidrug-resistant pathogens. They belong to the ribosomally synthesised and post-translationally modified peptide (RiPP) superfamily of natural products. The structure contains a unique four-amino acid macrocycle formed via a rare amidine linkage, C-methylation and a d-amino acid. This review covers all aspects of bottromycin research with a focus on recent years (2009–2020), in which major advances in total synthesis and understanding of bottromycin biosynthesis were achieved.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Natural Product Reports
化学-生化与分子生物学
CiteScore
21.20
自引率
3.40%
发文量
127
审稿时长
1.7 months
期刊介绍:
Natural Product Reports (NPR) serves as a pivotal critical review journal propelling advancements in all facets of natural products research, encompassing isolation, structural and stereochemical determination, biosynthesis, biological activity, and synthesis.
With a broad scope, NPR extends its influence into the wider bioinorganic, bioorganic, and chemical biology communities. Covering areas such as enzymology, nucleic acids, genetics, chemical ecology, carbohydrates, primary and secondary metabolism, and analytical techniques, the journal provides insightful articles focusing on key developments shaping the field, rather than offering exhaustive overviews of all results.
NPR encourages authors to infuse their perspectives on developments, trends, and future directions, fostering a dynamic exchange of ideas within the natural products research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


