中、高脂肪膳食对多替格拉韦/利匹韦林固定剂量联合片生物利用度的影响。
IF 2.5
Q2 PHARMACOLOGY & PHARMACY
Clinical Pharmacology : Advances and Applications
Pub Date : 2020-06-08
eCollection Date: 2020-01-01
DOI:10.2147/CPAA.S250751
引用次数: 3
摘要
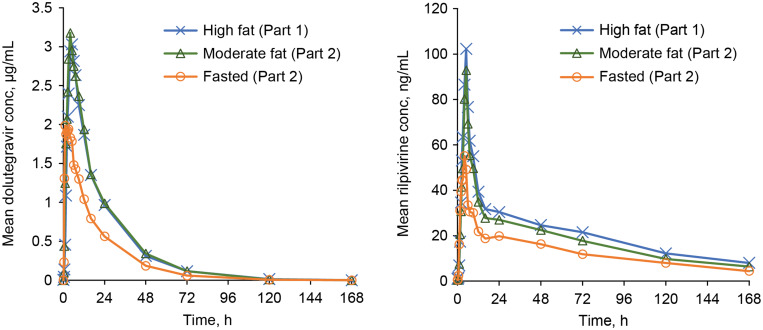
Dolutegravir 50mg (DTG)和rilpivirine 25mg (RPV)是一种新批准的治疗HIV病毒抑制患者的双药方案。本研究分为两部分,评价了五种DTG/RPV固定剂量联合片剂的相对生物利用度和食用效应。当给予中等或高脂肪膳食时,DTG和RPV的吸收都增加了,导致更高的暴露。根据产品标签,DTG/RPV / FDC应随餐服用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Effect of Moderate- and High-Fat Meals on the Bioavailability of Dolutegravir/Rilpivirine Fixed-Dose Combination Tablet.
Dolutegravir 50 mg (DTG) and rilpivirine 25 mg (RPV) are a newly approved 2-drug regimen for the treatment of HIV in virally suppressed patients. A 2-part study evaluated the relative bioavailability and food effect of five experimental fixed-dose combination (FDC) tablet formulations of DTG/RPV. When given with a moderate- or high-fat meal, the absorption of both DTG and RPV was increased, resulting in higher exposures. As per product labelling, DTG/RPV FDC should be taken with a meal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Clinical Pharmacology : Advances and Applications
PHARMACOLOGY & PHARMACY-
CiteScore
4.60
自引率
0.00%
发文量
14
审稿时长
16 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


