芳基环丙烷与水的电化学开环1,3-二羟基化反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
烯烃的常规二羟基化反应是在相邻位置传递两个羟基的最有效的合成手段之一。直接形成1,3-二醇仍然是一个艰巨的挑战,然而二羟基广泛存在于生物活性化合物中,目前只能通过多个步骤合成。芳基环丙烷的氧化开环已被证明可以获得各种1,3-二官能化的化学物质,但由于其固有的高倾向于进一步氧化,没有直接合成1,3-二醇。在此,我们报告了一种简单而有效的1,3-二醇策略,涉及控制芳基环丙烷的电化学C-C键裂解,以H2O作为最终的绿色羟基源。此外,该方案具有高原子经济性,广泛的底物范围和良好的官能团耐受性,因此适用于复杂的天然产物和药物衍生物的合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
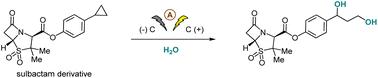
Electrochemical ring-opening 1,3-dihydroxylation of arylcyclopropanes with H2O†‡
Conventional dihydroxylation of alkenes is one of the most powerful synthetic tools for delivering two hydroxyl groups at vicinal positions. The direct formation of 1,3-diols remains a formidable challenge, yet dihydroxyl groups are broadly present in bioactive compounds, and are currently only available synthetically via multiple steps. The oxidative ring-opening of arylcyclopropanes has been demonstrated to access various 1,3-difunctionalized chemicals, but no 1,3-diols have been directly synthesized owing to their inherently high proclivity to become further oxidized. Herein, we report a facile and efficient strategy to 1,3-diols involving controlled electrochemical C–C bond cleavage of arylcyclopropanes with H2O as the ultimately green hydroxyl source. Moreover, this protocol stands out with its high atom economy, broad substrate scope and excellent functional group tolerance, and hence is amenable to the synthesis of complex natural products and drug derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


