芳基醚的光催化转移氢解†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
芳基醚对木质素解聚和木质素产物中的氧含量有很大影响。裂解芳醚通常需要恶劣的条件,如高压氢气和高温。在此,我们开发了一种协同方法,将光催化氢转移与酸催化相结合,在室温下进行二苯基醚和芳香氧合物的无h2氢解。富电子Pt/TiO2表面在光照射下储存了丰富的氢,并有效催化氢从异丙醇向芳基醚的转移。酸介导的加氢顺序为:芳基C-O键的氢解;芳基环的饱和:脂肪族C-O键的氢解。DFT计算表明,吸附在铂表面的芳基醚键通过质子化作用被削弱。该方法从二苯醚裂解得到98%的脂肪族单体(73%环己烷和25%环己醇),并在温和条件下将芳香族混合物转化为环烷烃(57%)和脂肪族醇(9%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
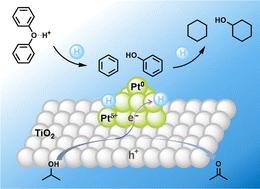
Photocatalytic transfer hydrogenolysis of aryl ethers†
Aryl ethers greatly influence lignin depolymerization and the oxygen content in lignin products. Cleaving aryl ethers normally requires harsh conditions such as high-pressure hydrogen gas and elevated temperature. Herein, we developed a synergistic method that combines photocatalytic hydrogen transfer with acid catalysis for H2-free hydrogenolysis of diphenyl ether and aromatic oxygenates at room temperature. The electron-enriched Pt/TiO2 surface stored abundant hydrogen species under light irradiation and efficiently catalyzed hydrogen transfer from isopropanol to aryl ethers. The acid mediated the hydrogenation sequence into: hydrogenolysis of aryl C–O bonds > saturation of aryl rings ≫ hydrogenolysis of aliphatic C–O bonds. DFT calculations suggested the aryl ether bond adsorbed on the Pt surface was weakened through protonation. This method delivered 98% yield of aliphatic monomers (73% cyclohexane and 25% cyclohexanol) from cleavage of diphenyl ether, and converted aromatic mixtures into cycloalkanes (57%) and aliphatic alcohols (9%) under mild conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


