可见光诱导碘化烷基与α-三氟甲基取代苯乙烯的脱氟羰基偶联
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了可见光介导的烷基碘化物与α-三氟甲基苯乙烯的脱氟羰基交叉偶联反应。该反应在室温下蓝光照射下进行,并以中高收率得到了各种宝石二氟烯烃。并对所得产物进行了合成转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
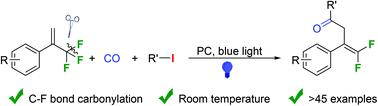
Visible-light-induced defluorinative carbonylative coupling of alkyl iodides with α-trifluoromethyl substituted styrenes†
A visible-light-mediated defluorinative carbonylative cross-coupling of alkyl iodides with α-trifluoromethyl styrenes has been developed. The reaction occurs at room temperature under blue light irradiation, and various gem-difluoroalkenes were obtained in moderate to good yields. Synthetic transformations of the obtained product were performed as well.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


