纳米结构的水笼细胞色素c与提高稳定性在恶劣环境:分子的洞察力†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
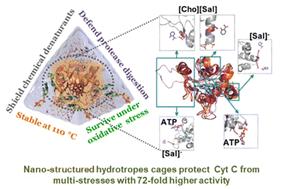
绿色和纳米结构的催化介质对生物催化至关重要,可以减轻生物催化剂在恶劣反应条件下的变性倾向。具有多方面理化性质的水变性物代表了可持续蛋白质包装的有前途的系统。其中,腺苷-5 ' -三磷酸腺苷(ATP)和水杨酸胆碱([Cho][Sal])离子液体(IL)形成纳米结构和纳米限制细胞色素c (Cyt c)的能力增强了在多种胁迫下的稳定性和活性。实验和计算分析解释了ATP和IL的纳米结构现象,纳米限制性Cyt c的结构组织,以及稳定蛋白质结构的位点特异性相互作用。ATP和IL在水介质中形成纳米结构,并通过多种非特异性软相互作用笼化Cyt c。值得注意的是,ATP (5-10 mM)、IL (300 mg mL - 1)和ATP + IL环绕Cyt c的纳米笼的过氧化物酶活性比天然Cyt c高9- 72倍,具有极高的耐热性(110°c)。与Cyt c的心磷脂结合位点的极性相互作用,由水异构物介导,与过氧化物酶活性的增加密切相关。此外,在尿素、GuHCl和胰蛋白酶存在的情况下,观察到更高的活性趋势,而没有任何蛋白质降解。在Cyt c的高流动区域(Ω 40-54残基)上的亲水物特异性结合以及与赖氨酸和精氨酸的h键增强,在极端条件下提供了出色的稳定性。此外,ATP有效地抵消了活性氧(ROS)诱导的Cyt c变性,而这种变性被IL的[Sal]对偶物增强。总的来说,本研究探索了纳米结构的水产物的稳健性,在极端条件下具有更高的稳定性和活性,具有更高的蛋白质包装潜力。因此,目前的工作强调了实时工业生物催化的新策略,以保护线粒体细胞免受ros诱导的凋亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nano-structured hydrotrope-caged cytochrome c with boosted stability in harsh environments: a molecular insight†
Green and nano-structured catalytic media are vital for biocatalysis to attenuate the denaturation tendency of biocatalysts under severe reaction conditions. Hydrotropes with multi-faceted physiochemical properties represent promising systems for sustainable protein packaging. Herein, the ability of adenosine-5′-triphosphate (ATP) and cholinium salicylate ([Cho][Sal]) ionic liquid (IL) to form nano-structures and to nano-confine Cytochrome c (Cyt c) enhanced the stability and activity under multiple stresses. Experimental and computational analyses were undertaken to explain the nano-structured phenomenon of ATP and IL, structural organizations of nano-confined Cyt c, and site-specific interactions that stabilize the protein structure. Both ATP and IL form nano-structures in aqueous media and could cage Cyt c via multiple nonspecific soft interactions. Remarkably, the engineered molecular nano-cages of ATP (5–10 mM), IL (300 mg mL−1), and ATP + IL surrounding Cyt c resulted in 9-to-72-fold higher peroxidase activity than native Cyt c with exceptionally high thermal tolerance (110 °C). The polar interactions with the cardiolipin binding site of Cyt c, mediated by hydrotropes, were well correlated with the increased peroxidase activity. Furthermore, higher activity trends were observed in the presence of urea, GuHCl, and trypsin without any protein degradation. Specific binding of hydrotropes in highly mobile regions of Cyt c (Ω 40–54 residues) and enhanced H-bonding with Lys and Arg offered excellent stability under extreme conditions. Additionally, ATP effectively counteracted reactive oxygen species (ROS)-induced denaturation of Cyt c, which was enhanced by the [Sal] counterpart of IL. Overall, this study explored the robustness of nano-structured hydrotropes to have a higher potential for protein packaging with improved stability and activity under extreme conditions. Thus, the present work highlights a novel strategy for real-time industrial biocatalysis to protect mitochondrial cells from ROS-instigated apoptosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


