临床适当使用达那韦800毫克。
IF 1.6
4区 医学
Q4 INFECTIOUS DISEASES
Southern African Journal of Hiv Medicine
Pub Date : 2018-10-18
eCollection Date: 2018-01-01
DOI:10.4102/sajhivmed.v19i1.918
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
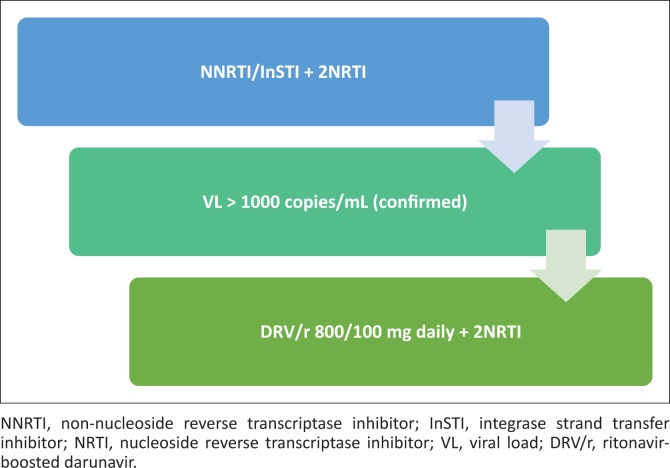
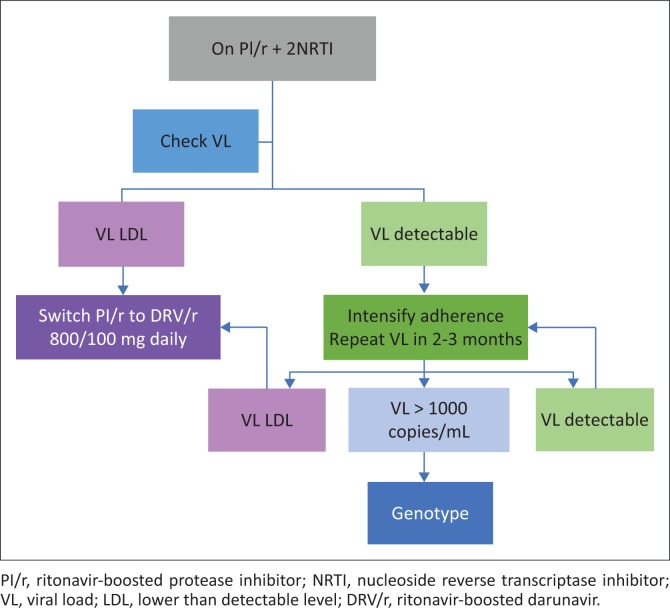
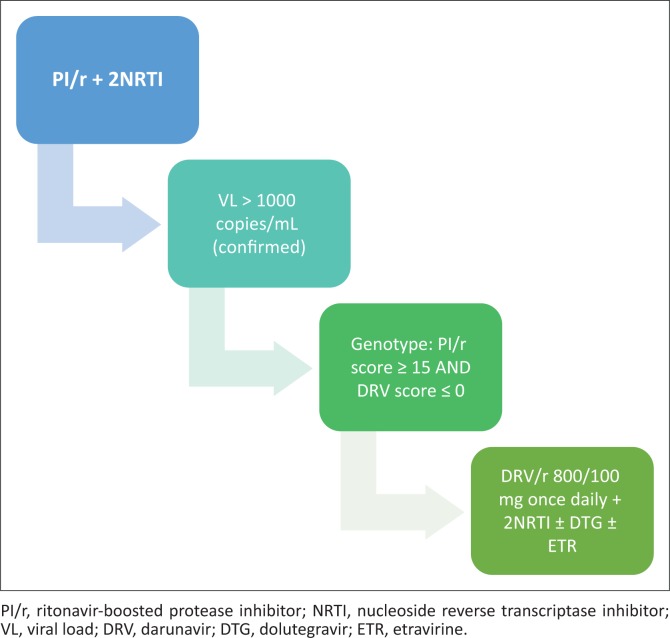
Appropriate clinical use of darunavir 800 mg.
PREZISTA, in combination with low dose ritonavir (DRV/r) and with other antiretroviral medicines, is indicated for the treatment of human immunodeficiency virus (HIV) infection in antiretroviral treatment experienced adult patients who are protease-inhibitor-naïve or after exclusion of darunavir resistance associated mutations (DRV-RAMs: V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V and L89V). Genotypic or phenotypic testing should guide the use of DRV/r. (Prezista package insert)
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
2.80
自引率
11.80%
发文量
41
审稿时长
>12 weeks
期刊介绍:
The Southern African Journal of HIV Medicine is focused on HIV/AIDS treatment, prevention and related topics relevant to clinical and public health practice. The purpose of the journal is to disseminate original research results and to support high-level learning related to HIV Medicine. It publishes original research articles, editorials, case reports/case series, reviews of state-of-the-art clinical practice, and correspondence.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


