dTAG 系统可实现蛋白质的即时和靶向降解
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 478
摘要
解剖复杂的生物系统需要对蛋白质的功能或丰度进行靶向控制。基因扰动受到脱靶效应、多组分复杂性和不可逆性的限制。最大的限制在于从调制到实验测量之间的必要延迟。为了实现对单一蛋白质丰度的即时和选择性控制,我们创建了一个化学生物学系统,利用细胞渗透性异功能降解剂的效力。dTAG 系统将 FKBP12F36V 的新型降解剂与 FKBP12F36V 与感兴趣的蛋白质同框表达配对。通过转基因表达或 CRISPR 介导的基因座特异性敲入,我们举例说明了研究蛋白质缺失直接后果的通用策略。利用 dTAG,我们观察到泛 BET 溴域降解比选择性 BRD4 降解具有意想不到的抗增殖效果,描述了 KRASG12V 缺失对蛋白质组信号转导的直接影响,并展示了体内的快速降解。该技术平台将为生物学研究提供动力学分辨率,并在药物发现方面提供靶点验证。dTAG 系统将强效的异功能降解剂和可扩展的标记策略配对使用,实现了不同蛋白质的即时和可逆降解,促进了细胞和小鼠中的生物学研究和药物靶点验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
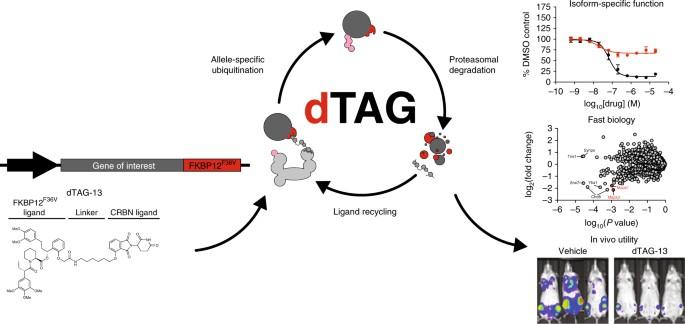
The dTAG system for immediate and target-specific protein degradation
Dissection of complex biological systems requires target-specific control of the function or abundance of proteins. Genetic perturbations are limited by off-target effects, multicomponent complexity, and irreversibility. Most limiting is the requisite delay between modulation to experimental measurement. To enable the immediate and selective control of single protein abundance, we created a chemical biology system that leverages the potency of cell-permeable heterobifunctional degraders. The dTAG system pairs a novel degrader of FKBP12F36V with expression of FKBP12F36V in-frame with a protein of interest. By transgene expression or CRISPR-mediated locus-specific knock-in, we exemplify a generalizable strategy to study the immediate consequence of protein loss. Using dTAG, we observe an unexpected superior antiproliferative effect of pan-BET bromodomain degradation over selective BRD4 degradation, characterize immediate effects of KRASG12V loss on proteomic signaling, and demonstrate rapid degradation in vivo. This technology platform will confer kinetic resolution to biological investigation and provide target validation in the context of drug discovery. The dTAG system pairs potent heterobifunctional degraders and extensible tagging strategies to achieve immediate and reversible degradation of divergent proteins, facilitating biological investigation and drug target validation in cells and in mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


