肿瘤外泌体可拮抗先天性抗病毒免疫力
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 133
摘要
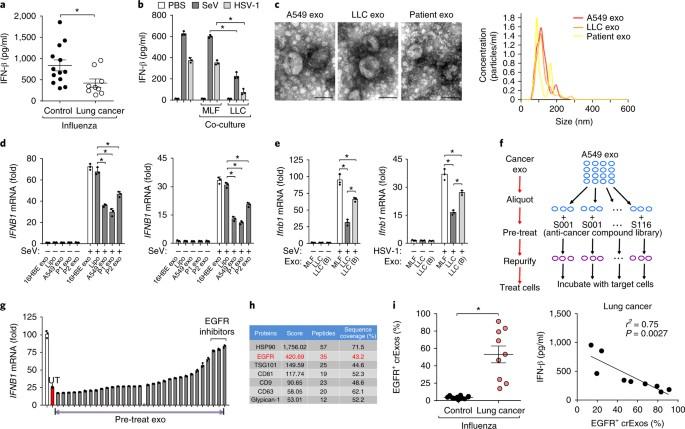
恶性肿瘤会损害先天性免疫,但其机制在很大程度上还不清楚。在这里,我们发现,通过肿瘤衍生外泌体(TEXs),癌症能够将活化的表皮生长因子受体(EGFR)转移到宿主巨噬细胞,从而抑制先天性抗病毒免疫。对人类激酶组的筛选发现,巨噬细胞中的激酶MEKK2是TEX传递表皮生长因子受体的效应器,它对抗病毒免疫反应有负面调节作用。在实验性肿瘤植入的情况下,MEKK2缺陷小鼠比野生型小鼠更能抵抗病毒感染。向小鼠注射TEX会降低先天免疫力,增加病毒载量,并以表皮生长因子受体和MEKK2依赖的方式增加发病率。MEKK2使IRF3磷酸化,IRF3是一种对产生I型干扰素至关重要的转录因子;这引发了IRF3的多泛素化,并阻断了它的二聚化、向细胞核的转位以及病毒感染后的转录活性。这些发现确定了癌细胞抑制宿主先天免疫并可能导致癌症患者免疫功能低下的机制。癌症可能与先天性免疫的改变有关。Gao 等人证明,这些改变源于肿瘤释放的免疫调节外泌体,这些外泌体抑制了巨噬细胞产生 I 型干扰素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumor-derived exosomes antagonize innate antiviral immunity
Malignancies can compromise innate immunity, but the mechanisms of this are largely unknown. Here we found that, via tumor-derived exosomes (TEXs), cancers were able to transfer activated epidermal growth factor receptor (EGFR) to host macrophages and thereby suppress innate antiviral immunity. Screening of the human kinome identified the kinase MEKK2 in macrophages as an effector of TEX-delivered EGFR that negatively regulated the antiviral immune response. In the context of experimental tumor implantation, MEKK2-deficient mice were more resistant to viral infection than were wild-type mice. Injection of TEXs into mice reduced innate immunity, increased viral load and increased morbidity in an EGFR- and MEKK2-dependent manner. MEKK2 phosphorylated IRF3, a transcription factor crucial for the production of type I interferons; this triggered poly-ubiquitination of IRF3 and blocked its dimerization, translocation to the nucleus and transcriptional activity after viral infection. These findings identify a mechanism by which cancer cells can dampen host innate immunity and potentially cause patients with cancer to become immunocompromised. Cancer can be associated with alterations in innate immunity. Gao et al. demonstrate that these alterations arise from immunomodulatory exosomes released by tumors, which then dampen the production of type I interferons by macrophages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


