用 mRNA 编码的双特异性抗体消除小鼠体内的大肿瘤
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 156
摘要
连接 T 细胞和肿瘤细胞的双特异性抗体可以体外转录的药理学优化 mRNA 的形式递送;将这些 mRNA 编码的抗体注射到小鼠体内后,其排斥大型肿瘤的效率与相应的重组抗体蛋白相当。双特异性 T 细胞诱导抗体的潜力受到生产难题和血清半衰期短的阻碍。我们用体外转录的经过药理学优化、核苷修饰的编码抗体的 mRNA 处理小鼠,从而规避了这些限制。我们实现了抗体的持续内源性合成,与相应的纯化双特异性抗体一样有效地消除了晚期肿瘤。由于药用 mRNA 的制造速度很快,这种方法可以加速新型双特异性抗体的临床开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
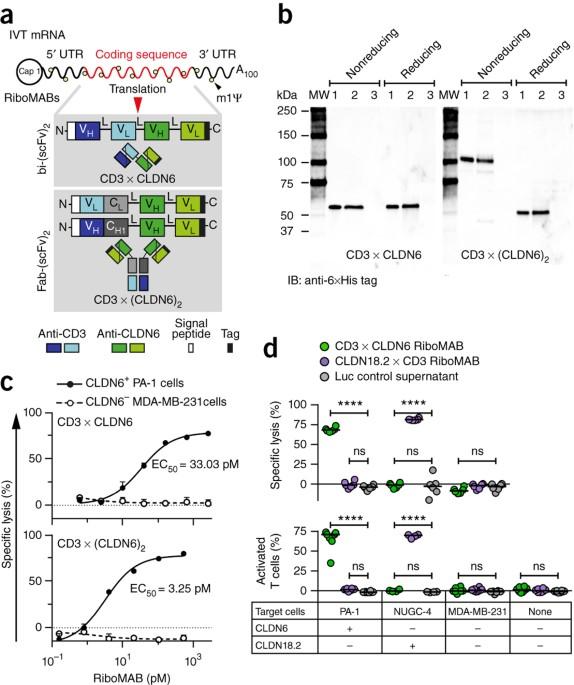
Elimination of large tumors in mice by mRNA-encoded bispecific antibodies
Bispecific antibodies that connect T cells with tumor cells can be delivered in the form of in vitro–transcribed pharmacologically optimized mRNA; when injected into mice, these mRNA-encoded antibodies reject large established tumors as efficiently as the corresponding recombinant antibody protein. The potential of bispecific T cell–engaging antibodies is hindered by manufacturing challenges and short serum half-life. We circumvented these limitations by treating mice with in vitro–transcribed pharmacologically optimized, nucleoside-modified mRNA encoding the antibody. We achieved sustained endogenous synthesis of the antibody, which eliminated advanced tumors as effectively as the corresponding purified bispecific antibody. Because manufacturing of pharmaceutical mRNA is fast, this approach could accelerate the clinical development of novel bispecific antibodies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


