铂(II)磺胺配合物的生物学评价:合成、表征、细胞毒性和生物成像。
IF 4.1
3区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Bioinorganic Chemistry and Applications
Pub Date : 2022-09-13
eCollection Date: 2022-01-01
DOI:10.1155/2022/7821284
引用次数: 2
摘要
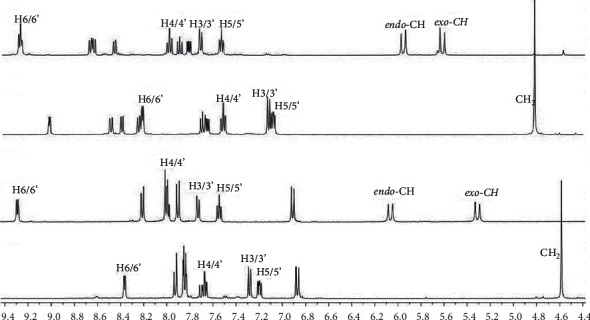
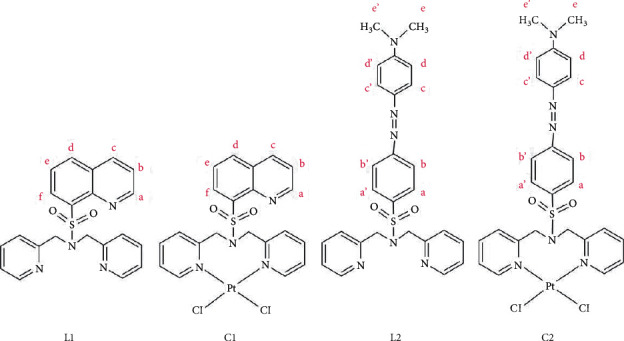
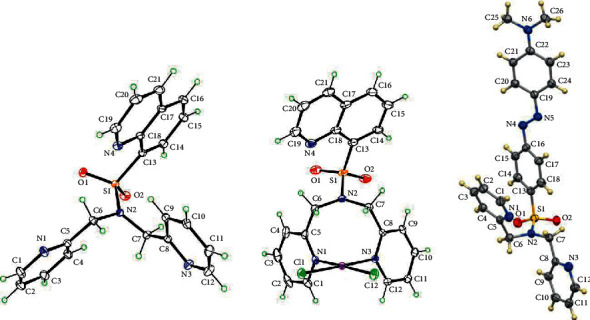
铂基化合物作为抗癌药物积极应用于临床试验。本研究以较好的收率合成了两种新型的含喹啉和偶氮苯二聚胺磺酰胺配体的铂配合物(C1 = [PtCl2(N(SO2quin)dpa)], C2 = [PtCl2(N(SO2quin)dpa)]。C1和C2配体的亚甲基CH2质子的单线态在1H NMR谱中表现为两个双线态,证实了与铂配位时存在磁性不等效质子。N(SO2quin)dpa (L1)、N(so2偶氮苯)dpa (L2)和PtCl2(N(SO2quin)dpa)的结构数据证实了所需化合物的形成。时间依赖的密度泛函理论计算表明,L1的激发表现为基于quin-单位的π(即配体中心电荷转移,LC),而C1表现为金属-配体到配体的电荷转移(MLLCT)特征。L1在1LC激发态下发出强烈荧光,而C1在3LC激发态下发出磷光。用NCI-H292非小细胞肺癌细胞评估配体和复合物的哺乳动物细胞毒性。此外,C1和C2的IC50值与N(SO2azobenz)dpa和PtCl2(N(SO2quin)dpa相比显著降低。配体和配合物的荧光成像数据显示了这些化合物在生物成像方面的潜在荧光活性。这四种化合物都是很有前景的新候选物,可以进一步研究它们作为潜在的抗癌剂和癌细胞显像剂的用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Biological Evaluation of Platinum(II) Sulfonamido Complexes: Synthesis, Characterization, Cytotoxicity, and Biological Imaging.
Platinum-based compounds are actively used in clinical trials as anticancer agents. In this study, two novel platinum complexes, (C1 = [PtCl2(N(SO2quin)dpa)], C2 = [PtCl2(N(SO2azobenz)dpa)]) containing quinoline and azobenzene appended dipicolylamine sulfonamide ligands were synthesized in good yield. The singlet attributable to methylene CH2 protons of the ligands of C1 and C2 appears as two doublets in 1H NMR spectra, which confirms the presence of magnetically nonequivalent protons upon coordination to platinum. Structural data of N(SO2quin)dpa (L1), N(SO2azobenz)dpa (L2) and PtCl2(N(SO2quin)dpa) confirmed the formation of the desired compounds. Time-dependent density functional theory calculations suggested that the excitation of L1 show quin-unit-based π⟶π∗ excitations (i.e., ligand-centered charge transfer, LC), while C1 shows the metal-ligand-to-ligand charge-transfer (MLLCT) character. L1 displays intense fluorescence from the 1LC excited state, while C1 gives phosphorescence from the 3LC state. Mammalian cell toxicity of ligands and complexes was assessed with NCI–H292 nonsmall-cell lung cancer cells. Further, C1 and C2 showed significantly low IC50 values compared with N(SO2azobenz)dpa and PtCl2(N(SO2quin)dpa). Fluorescence imaging data of both ligands and complexes revealed the potential fluorescence activity of these compounds for biological imaging. All four compounds are promising novel candidates that can be further investigated on their usage as potential anticancer agents and cancer cell imaging agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioinorganic Chemistry and Applications
化学-生化与分子生物学
CiteScore
7.00
自引率
5.30%
发文量
105
审稿时长
>12 weeks
期刊介绍:
Bioinorganic Chemistry and Applications is primarily devoted to original research papers, but also publishes review articles, editorials, and letter to the editor in the general field of bioinorganic chemistry and its applications. Its scope includes all aspects of bioinorganic chemistry, including bioorganometallic chemistry and applied bioinorganic chemistry. The journal welcomes papers relating to metalloenzymes and model compounds, metal-based drugs, biomaterials, biocatalysis and bioelectronics, metals in biology and medicine, metals toxicology and metals in the environment, metal interactions with biomolecules and spectroscopic applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


