受精卵细胞分泌内肽酶,以避免多聚酶的产生
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 29
摘要
配子融合时,动物卵细胞从皮质颗粒中分泌蛋白酶,形成受精包膜,阻碍多精子受精1-4。有花植物的受精过程更为复杂,需要花粉管输送两个无运动能力的精子细胞5,6。通常要避免多个花粉管同时穿透胚珠(多管受精),从而间接地防止多精子症7,8。植物卵细胞如何在成功受精后调节多余花粉管的排出尚不清楚。在这里,我们报告了天冬氨酸内肽酶 ECS1 和 ECS2 从位于拟南芥卵细胞顶端的皮层网络分泌到细胞外空间。这种反应只有在成功受精后才会触发。ECS1 和 ECS2 只在卵细胞中表达,配子融合后转录物立即降解。ECS1 和 ESC2 专门裂解花粉管吸引子 LURE1。因此,ecs1 ecs2 双突变体中经常出现多管现象。协同体细胞异位分泌这些内肽酶会导致 LURE1 水平下降,并降低花粉管吸引率。这些发现共同证明了植物卵细胞能感知成功的受精,并阐明了受精后如何通过受精诱导的吸引因子降解来相对快速地阻断多花粉管的机制。受精的拟南芥卵细胞向细胞外空间分泌内肽酶,裂解花粉管吸引因子 LURE1,阻止多管现象。本文章由计算机程序翻译,如有差异,请以英文原文为准。
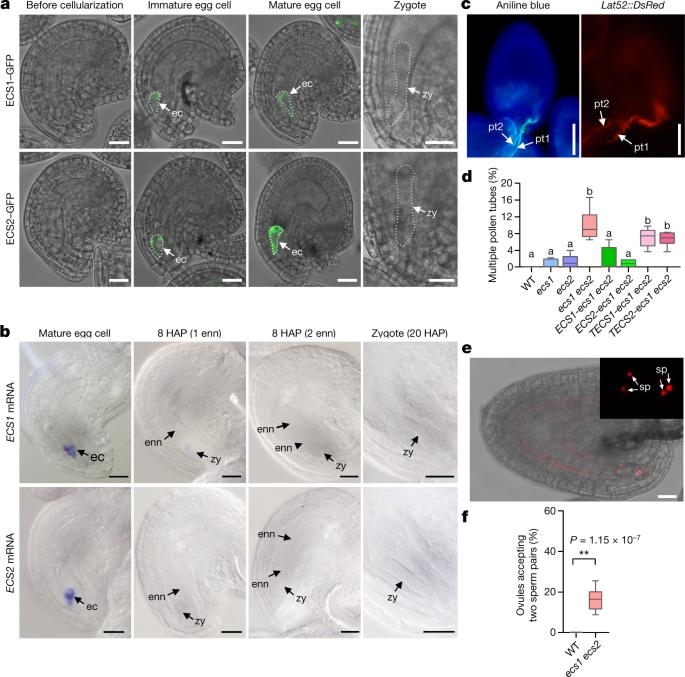
Fertilized egg cells secrete endopeptidases to avoid polytubey
Upon gamete fusion, animal egg cells secrete proteases from cortical granules to establish a fertilization envelope as a block to polyspermy1–4. Fertilization in flowering plants is more complex and involves the delivery of two non-motile sperm cells by pollen tubes5,6. Simultaneous penetration of ovules by multiple pollen tubes (polytubey) is usually avoided, thus indirectly preventing polyspermy7,8. How plant egg cells regulate the rejection of extra tubes after successful fertilization is not known. Here we report that the aspartic endopeptidases ECS1 and ECS2 are secreted to the extracellular space from a cortical network located at the apical domain of the Arabidopsis egg cell. This reaction is triggered only after successful fertilization. ECS1 and ECS2 are exclusively expressed in the egg cell and transcripts are degraded immediately after gamete fusion. ECS1 and ESC2 specifically cleave the pollen tube attractor LURE1. As a consequence, polytubey is frequent in ecs1 ecs2 double mutants. Ectopic secretion of these endopeptidases from synergid cells led to a decrease in the levels of LURE1 and reduced the rate of pollen tube attraction. Together, these findings demonstrate that plant egg cells sense successful fertilization and elucidate a mechanism as to how a relatively fast post-fertilization block to polytubey is established by fertilization-induced degradation of attraction factors. Fertilized Arabidopsis egg cells secrete endopeptidases into the extracellular space that cleave the pollen tube attractor LURE1, preventing polytubey.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


