铜介导的2h -茚唑与n -氟苯磺酰亚胺的CH胺化反应
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文以市售的n -氟苯磺酰亚胺(NFSI)为胺化试剂,建立了一种铜催化的2h -吲哚C-3位胺化反应。结果表明,在温和的条件下,衬底范围广,产率中等至良好。所描述的过程涉及一个自由基加成过程,从而形成新的CN键,它为合成功能化2h -吲哚提供了一种新的方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
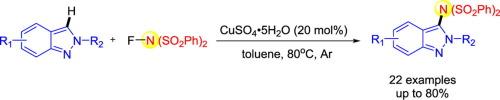
Copper-mediated CH amination of 2H-indazoles with N–Fluorobenzenesulfonimide
A facile copper-catalyzed amination in C-3 position of 2H-indazoles using commercially available N-fluorobenzenesulfonimide (NFSI) as an amidating reagent was established in this paper. The results revealed broad substrate scope in moderate to good yields under mild conditions. The described procedure involves a radical addition process whereby new CN bonds are formed, and it provides a new protocol for the synthesis of functionalized 2H-indazoles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


