立体控制α-氨基酸n -芳基化合成PI3K抑制剂GDC-0077
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 14
摘要
报道了一种高效合成PI3K抑制剂GDC-0077的方法,该方法具有两个连续的cu催化CN偶联反应。所描述的合成路线包括手性二氟甲基取代的恶唑烷酮的化学选择性ullmann型偶联,具有高立体化学完整性的cu催化的l-丙氨酸n-芳基化,以及高收率的最终酰胺键形成步骤,以生产纯度为99.5%的GDC-0077。本文章由计算机程序翻译,如有差异,请以英文原文为准。
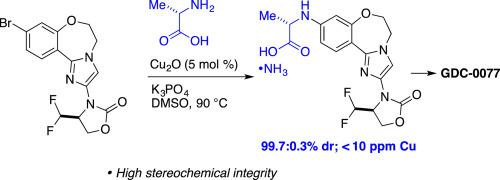
Synthesis of PI3K inhibitor GDC-0077 via a stereocontrolled N-arylation of α-amino acids
An efficient synthesis of PI3K inhibitor GDC-0077, featuring two consecutive Cu-catalyzed CN coupling reactions, is reported. The described synthetic route involves a chemoselective Ullmann-type coupling of a chiral difluoromethyl-substituted oxazolidinone, a Cu-catalyzed N-arylation of l-alanine with high stereochemical integrity, and a high-yielding final amide bond formation step to produce GDC-0077 in >99.5 area % HPLC purity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


