丙炔醇与磺酰肼经TBHP/ tbai介导的反应合成烯基砜
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 60
摘要
在HOAc的存在下,建立了一种新的由TBHP/ tbai介导的丙炔醇与磺酰肼的反应,在此反应中,以中高收率得到了多种烯基砜。机理研究表明,这种转化包括hoac促进的磺酰肼中间产物的形成,顺序的C-O、C-N和N-S键的裂解,以及C-S键的形成。值得注意的是,这种磺化反应是在自由基过程中进行的,具有高度的官能团相容性和优异的区域选择性,反应时间短,试剂价格便宜。本文章由计算机程序翻译,如有差异,请以英文原文为准。
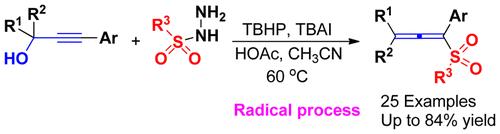
Synthesis of Allenyl Sulfones via a TBHP/TBAI-Mediated Reaction of Propargyl Alcohols with Sulfonyl Hydrazides
A new TBHP/TBAI-mediated reaction of propargyl alcohols with sulfonyl hydrazides in the presence of HOAc has been established, in which a wide variety of allenyl sulfones were obtained in moderate to excellent yields. Mechanistic studies indicate that this transformation involves HOAc-promoted sulfonohydrazide intermediate formation, sequential C–O, C–N, and N–S bond cleavage, and C–S bond formation. Significantly, this sulfonohylation proceeds in a radical process and shows highly functional group compatibility and excellent regioselectivity, with a short reaction time and inexpensive reagents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


