酰基唑盐的Norrish II型反应
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 3
摘要
研究了由脂肪族羧酸衍生的酰基唑盐的光化学反应性。这些物种作为n -杂环碳(NHC)有机催化生成中间体的模型,在UVA光照射下进行Norrish II型消除反应,类似于结构相关的芳香酮。此外,从金刚烷基取代衍生物中观察到有效的诺里-杨环化。这些结果进一步证明了NHCs在催化循环中影响羰基的吸收特性和光化学反应活性的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
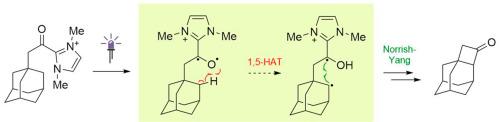
Norrish type II reactions of acyl azolium salts
The photochemical reactivity of acyl azolium salts derived from aliphatic carboxylic acids has been investigated. These species, which serve as models for intermediates generated in N-heterocyclic carbene (NHC) organocatalysis, undergo Norrish type II elimination reactions under irradiation with UVA light in analogy to structurally related aromatic ketones. Moreover, efficient Norrish-Yang cyclization was observed from an adamantyl-substituted derivative. These results further demonstrate the ability of NHCs to influence the absorption properties and photochemical reactivity of carbonyl groups during a catalytic cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


