锰-52标记环糊精的临床前评价:前列腺素E2仿射成像探针。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
前列腺素E2 (PGE2)参与多种肿瘤相关的生物学过程,是一种很有前景的分子成像生物标志物。在核医学中,放射性标记的环糊精已成为各种癌症类型中靶向PGE2的潜在探针。随后,我们旨在通过多时间点正电子发射断层扫描和PGE2阳性BxPC-3胰腺腺癌异种移植物的体外生物分布研究,确定一种新型锰-52标记的随机甲基化β-环糊精([52Mn]Mn-DOTAGA-RAMEB)的药代动力学。与离体研究结果一致,[52Mn]Mn-DOTAGA-RAMEB在早期和特异性肿瘤摄取(注射后30和60分钟),随后快速冲洗和肾脏清除。我们的实验数据证明了52mn标记的RAMEB环糊精识别表达PGE2的肿瘤的可行性,尽管需要进一步的药代动力学优化以提高肿瘤保留率并充分发挥其诊断潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
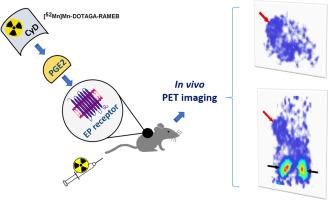
Preclinical evaluation of Manganese-52 labelled cyclodextrin: a prostaglandin E2 affine imaging probe
Prostaglandin E2 (PGE2) is implicated in several tumor-related biological processes, making it a promising biomarker for molecular imaging. In nuclear medicine, radiolabelled cyclodextrins have emerged as potential probes to target PGE2 in various cancer types. Hereafter, we aimed to specify the pharmacokinetics of a novel Manganese-52-labelled randomly methylated β-cyclodextrin ([52Mn]Mn-DOTAGA-RAMEB) by performing multiple time-point positron emission tomography and ex vivo biodistribution studies in PGE2 positive BxPC-3 pancreatic adenocarcinoma xenografts. In line with the ex vivo findings, [52Mn]Mn-DOTAGA-RAMEB showed early and specific tumor uptake (30 and 60 min after injection), followed by rapid wash-out and renal clearance. Our experimental data demonstrate the feasibility of 52Mn-labelled RAMEB cyclodextrin to identify PGE2 expressing tumors, although further pharmacokinetic optimization is required to enhance tumor retention and fully exploit its diagnostic potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


