评估环丙沙星在水生环境中的发生和生态毒理学:对粘土基吸附修复措施的见解。
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
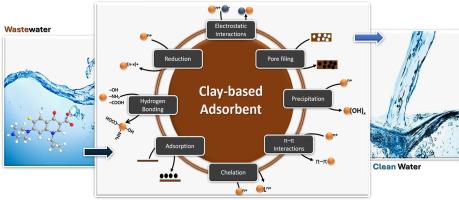
环丙沙星(CIP)由于其持续和大量使用,已成为引起环境关注的新兴污染物之一。对CIP的关注归因于其在环境中的持久性和不完全降解,这可能导致耐药菌株的传播。CIP的吸附在含CIP(废)水的处理中发挥了显著的作用,而粘土吸附剂的作用也日益显现。然而,与使用其他吸附剂相比,使用粘土基吸附剂进行CIP吸附的研究报道有限。其次,缺乏对粘土相互作用途径和有效性的了解可能会限制其在废水处理中的实际应用。鉴于这一差距,本文介绍了粘土基吸附剂吸附CIP的最新进展。研究结果表明,氧化石墨烯-高岭土复合材料和活性蒙脱石-高岭土复合材料对CIP吸附具有协同效果,吸附量分别为408.16 mg/g和344.82 mg/g。膨润土基复合材料的去除率最高,其中ZnO/ cuo -膨润土的实验去除率为451 mg/g,计算去除率约为1249.3 mg/g,去除率接近99%。总的来说,pH在控制CIP吸附中起主导作用,而静电吸引是主要的吸收机制。等温线模型表明Langmuir等温线(r2>0.96-0.99)是最合适的模型,证实了化学吸附是主要的机理。这种最先进的合成方法建议采用粘土和粘土复合材料进行进一步的跨学科研究,以优化可能的实际应用的吸附系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Assessing the occurrence and ecotoxicology of ciprofloxacin in aquatic environments: Insights into clay-based adsorptive remediation measures
Ciprofloxacin (CIP) is one of the emerging contaminants of environmental concern because of its continual and heavy use. Concerns about CIP are ascribed to its persistence and incomplete degradation in the environment, which could lead to the spread of drug-resistant bacterial strains. Adsorption of CIP has played a remarkable role in the treatment of CIP-laden (waste)water, and the role of clay adsorbents has increasingly emerged. However, limited studies have been reported on the use of clay-based adsorbents for CIP adsorption, compared to the use of other adsorbents. Second, the lack of understanding of interaction pathways and the effectiveness of clays might limit its practical application in wastewater treatment. Given this gap, this paper present state-of-the-art review on the adsorption of CIP using clay-based adsorbents. Our findings revealed that composite of graphene oxide–kaolinite and activated montmorillonite–kaolin are synergistically effective to remove CIP sorption with demonstrated sorption capacities of 408.16 mg/g and 344.82 mg/g, respectively. Bentonite-based composites showed the best performance, particularly ZnO/CuO–bentonite, with 451 mg/g experimentally and about 1249.3 mg/g calculated capacity, with a removal efficiency of nearly 99 %. Overall, pH was found to play dominant role in controlling CIP adsorption as well as the electrostatic attraction as dominant mechanism of uptake. Isotherm modelling suggested that the best fit model was Langmuir isotherm ( which confirms chemisorption as the dominant mechanism. This state-of-the-art synthesis recommends the adoption of clay and clay-composites for further interdisciplinary research in order to optimize adsorption systems for probable real-world applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


