Clethodim合成反应动力学研究及热危害评价
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
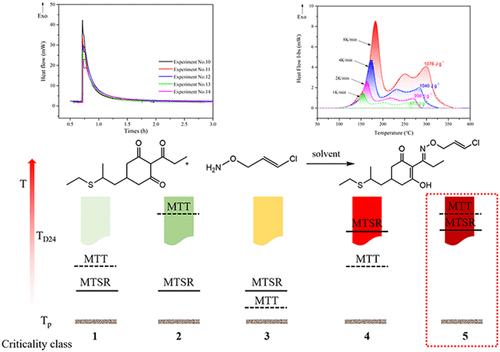
聚硫醚合成最后冷凝步骤的热失控造成了严重的工业事故(如2020年辽宁爆炸,造成5人死亡)。本研究重点研究了该步骤的热力学、动力学和热稳定性,以确保工艺安全。反应量热法测定反应热,取ΔrHm,A = 45000 J mol-1,绝热温升ΔTad = 35 K。测定了反应动力学参数,活化能Ea为47,000 J mol-1,指前因子k为17,185 L mol-1 s-1,反应符合二级动力学。差示扫描量热法和AKTS软件显示,CPHA和clethodim具有较高的热分解风险,其TD24值分别为65和75°C,而EPCO在300°C以下稳定。风险评价(TP、MTSR、MTT、TD24)结果显示,正常半批喂的危险临界水平为2,一次性批喂的危险临界水平为5,属于高风险。但是,如果混合良好,如果考虑到达到TD24所需的时间,风险也会得到控制。2020年的事故是由进料错误和局部过热(没有混合/传热)引起的。本研究为铅铅生产提供了动力学数据和安全措施。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinetic Study and Thermal Hazard Assessment of the Clethodim Synthesis Reaction
Thermal runaway in the final condensation step of the clethodim synthesis has caused severe industrial accidents (e.g., the 2020 Liaoning explosion, with 5 deaths). This study focused on the thermodynamics, kinetics, and thermal stability of this step to ensure process safety. Reaction calorimetry was used to determine the reaction heat, with ΔrHm,A = 45,000 J mol–1 and adiabatic temperature rise ΔTad = 35 K. The kinetic parameters were determined, revealing an activation energy Ea of 47,000 J mol–1, a pre-exponential factor k of 17,185 L mol–1 s–1, and the reaction followed second-order kinetics. Differential Scanning Calorimetry and AKTS software revealed that CPHA and clethodim have high thermal decomposition risks, with TD24 values of 65 and 75 °C, respectively, while EPCO was stable below 300 °C. Risk assessment (TP, MTSR, MTT, TD24) showed that the hazard criticality level of normal semibatch feeding was 2, and that of one-time batch feeding was 5, which was of high risk. However, with good mixing, if the time needed to reach TD24 was considered, the risk would also be under control. The 2020 accident was caused by feed errors and local overheating (no mixing/heat transfer). This work provides kinetic data and safety measures for clethodim production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


