乙酰丙酮的光化学:微滴介导的H2O2生成和SO2氧化
IF 3.7
2区 环境科学与生态学
Q2 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
h2o2驱动的氧化被认为是雾霾事件中硫酸盐形成的主要原因。然而,现场观测证实,已知的H2O2来源无法解释其环境水平。本研究利用吸湿性硫酸铵(NH4)2SO4和磷酸二氢钠(NaH2PO4)原位潮解形成的液滴,研究了大气中普遍存在的β-二酮乙酰丙酮(AA)在紫外(UV)照射下的光化学反应。特别强调阐明其在不同条件下对H2O2形成和SO2氧化的贡献。结果表明,AA光解能显著提高H2O2产量。在(NH4)2SO4液滴中,H2O2在紫外线照射后较早达到峰值,而在NaH2PO4液滴中,由于磷酸盐促进AA光解,H2O2达到了4倍的峰值,并且峰值较晚。像柠檬酸这样的氢原子供体将H2O2的自由基终止指向有机氢过氧化物(rohs)。Cl−和Br−改变了自由基过程:Cl−抑制H2O2的产生,但增强了ROOHs的产生,而Br−对两种物种的影响更强,且依赖于浓度。SO2的氧化是由AA光解过程中产生的H2O2和rohs等多种氧化物质实现的。鉴定了丙酮酸、过氧乙酸和丙二酸等关键产物,并揭示了可能的紫外驱动反应途径。这些结果揭示了新的H2O2生成和硫酸盐形成途径,并显示了气溶胶相光化学的复杂性。这项工作提高了我们对大气H2O2来源和氧化还原过程的认识,为大气有机气溶胶的氧化势和大气氧化能力提供了新的见解,并增强了大气化学模型,以更好地预测空气质量和气候。本文章由计算机程序翻译,如有差异,请以英文原文为准。
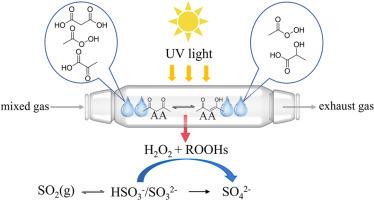
Photochemistry of acetylacetone: Droplets-mediated H2O2 generation and SO2 oxidation
H2O2-driven oxidation has been considered to be the main cause of sulfate formation in haze events. However, field observations have confirmed that the known H2O2 sources cannot explain its ambient levels. In this study, the photochemistry of acetylacetone (AA), a ubiquitous β-diketone in the atmosphere, was investigated under ultraviolet (UV) irradiation using droplets formed in-situ by deliquescence of hygroscopic ammonium sulfate ((NH4)2SO4) and sodium dihydrogen phosphate (NaH2PO4). Special emphasis was placed on elucidating its contributions to H2O2 formation and SO2 oxidation under varying conditions. Results show that AA photolysis significantly boosted H2O2 production. In (NH4)2SO4 droplets, H2O2 peaked early after UV irradiation, while in NaH2PO4 droplets, it reached fourfold higher and peaked later because phosphate promotes the AA photolysis. Hydrogen atom donors like citric acid redirected radical termination from H2O2 to organic hydroperoxides (ROOHs). Cl− and Br− altered radical processes: Cl− suppressed H2O2 production but enhanced ROOHs production, while Br− exhibits stronger, concentration-dependent effects on both species. SO2 oxidation was achieved by various oxidizing species such as H2O2 and ROOHs produced during AA photolysis. Key products such as pyruvic acid, peroxyacetic acid and propanedioic acid were identified and possible UV-driven reaction pathways were revealed. These results uncover new H2O2 production and sulfate formation pathways, and show the complexity of aerosol-phase photochemistry. This work improves our understanding of atmospheric H2O2 source and redox processes, offering new insights for the oxidation potential of atmospheric organic aerosols and atmospheric oxidation capacity, and enhancing atmospheric chemistry models to better predict air quality and climate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Atmospheric Environment
环境科学-环境科学
CiteScore
9.40
自引率
8.00%
发文量
458
审稿时长
53 days
期刊介绍:
Atmospheric Environment has an open access mirror journal Atmospheric Environment: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atmospheric Environment is the international journal for scientists in different disciplines related to atmospheric composition and its impacts. The journal publishes scientific articles with atmospheric relevance of emissions and depositions of gaseous and particulate compounds, chemical processes and physical effects in the atmosphere, as well as impacts of the changing atmospheric composition on human health, air quality, climate change, and ecosystems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


