超越峰值温度:生物质热解的传统与有效热力学模型的实验评估
IF 6.2
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
热重数据的准确解释取决于所使用的热力学模型,然而标准方法的实验比较是有限的。本研究对传统的(CA)和有效的热力学(ETA)方法进行了批判性评价,以分析甘蔗渣(SB)的热解过程。两种模型均证实SB热解是可行的、吸热的;但是,由于理论基础的不同,它们在熵(ΔS)和吉布斯自由能(ΔG)上产生了很大的差异。CA依赖于单峰温度(Tp),不能代表热解的多阶段性质。相比之下,ETA使用与转化相关的温度(Tα),更准确地捕获反应过程和生物量异质性。与其他生物量的比较表明,CA在Tp以下持续低估ΔS和高估ΔG,在Tp以上趋势逆转,而ETA提供了一致且有机械意义的趋势。尽管两种模型的焓值相似(ΔH),但ETA揭示了在高转化率下放热性增加和自发性降低的热力学转变(α > 0.35),这可能是由于SB的高萃取物含量(≈23%)和二次炭化反应——CA遗漏的信息。总体而言,模型选择强烈影响热力学解释,ETA被证明是设计和优化复杂、富含萃取物的热解的最佳方法。非采掘原料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
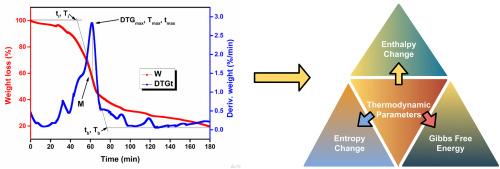
Beyond the peak temperature: An experimental evaluation of conventional vs. effective thermodynamic models for biomass pyrolysis
Accurate interpretation of thermogravimetric data depends on the thermodynamic model used, yet experimental comparisons of standard methods are limited. This study critically evaluates the conventional (CA) and effective thermodynamic (ETA) approaches for analyzing the pyrolysis of Sotol bagasse (SB), an extractive-rich agro-industrial residue. Both models confirmed that SB pyrolysis is feasible and endothermic; however, they produced substantial differences in entropy (ΔS) and Gibbs free energy (ΔG) due to their theoretical foundations. The CA relies on a single peak temperature (Tp), which fails to represent the multi-stage nature of pyrolysis. In contrast, the ETA uses conversion-dependent temperatures (Tα), capturing reaction progression and biomass heterogeneity more accurately. Comparisons with other biomasses showed that CA consistently underestimates ΔS and overestimates ΔG below Tp, with the trend reversing above Tp, whereas ETA provided consistent and mechanistically meaningful trends. Although both models yielded similar enthalpy (ΔH), the ETA revealed a thermodynamic shift toward increased exothermicity and reduced spontaneity at higher conversions (α > 0.35), likely due to SB's high extractive content (≈23 %) and secondary charring reactions—information missed by CA. Overall, model selection strongly influences thermodynamic interpretation, and the ETA is validated as the superior approach for designing and optimizing pyrolysis of complex, extractive-rich, and non-extractive feedstocks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


