人opn衍生肽SV通过与KIF5B相互作用,修复线粒体损伤,抑制细胞凋亡,从而预防BPD模型
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
母乳中的生物活性成分为支气管肺发育不良(BPD)提供了新的治疗策略。虽然骨桥蛋白(OPN)被添加到配方奶粉中以促进新生儿生长,但其衍生肽SVVYGLR (SV)在BPD中的治疗作用仍未被探索。本研究旨在阐明SV治疗BPD的机制。结果显示,SV可显著提高细胞活力,抑制高氧/ lps诱导的细胞凋亡,修复肺泡上皮损伤。分子上,SV恢复线粒体完整性,上调抗凋亡蛋白BCL-2,下调促凋亡蛋白Bax。体内实验表明,SV减少了高氧/脂多糖引发的bpd样损伤,新生儿小鼠体重增加,肺组织学改善,肺泡计数和间隔厚度增加。初步的机制研究表明SV与KIF5B相互作用并增强其与线粒体的结合。综上所述,SV通过KIF5B相互作用修复线粒体损伤并抑制细胞凋亡,从而改善BPD。本研究确定了BPD治疗的新的潜在靶点和治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
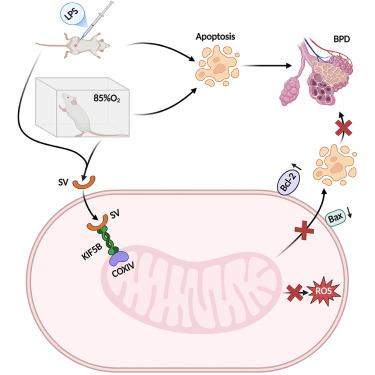
Human OPN-derived peptide SV prevents BPD model through interacting with KIF5B to repair mitochondrial damage and inhibit apoptosis
Bioactive components in breast milk offer a new therapeutic strategy for bronchopulmonary dysplasia (BPD). While osteopontin (OPN) is supplemented in formula to promote neonatal growth, the therapeutic role of its derived peptide, SVVYGLR (SV), in BPD remains unexplored. This study aimed to elucidate SV’s therapeutic mechanisms in BPD. The results revealed that SV significantly enhances cell viability, inhibits hyperoxia/LPS-induced apoptosis, and repairs alveolar epithelial injury. Molecularly, SV restores mitochondrial integrity, upregulates the anti-apoptotic protein BCL-2, and downregulates the pro-apoptotic protein Bax. In vivo experiments showed SV reduced hyperoxia/LPS-triggered BPD-like injury, evidenced by neonatal mice gaining weight, improved lung histology, and increased alveolar counts and septal thickness. Preliminary mechanistic studies revealed that SV interacts with KIF5B and enhances its binding to mitochondria. In conclusion, SV improves BPD by repairing mitochondrial damage and inhibiting apoptosis via KIF5B interaction. This study identified new potential targets and therapeutic strategies for BPD management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


