人脑膜中动脉周围的脑膜淋巴结构和引流动力学
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
脑脊液和间质液沿人腹侧脑膜清除的解剖学基础仍然不清楚。在这里,我们提出了体内和离体证据,证明在人脑膜中动脉(MMA)周围存在明显的CSF-ISF引流室。在5名健康参与者中,动态对比增强MRI显示mma周围区域的延迟信号增强,在90分钟达到峰值,大大晚于邻近的血管和淋巴结构,与较慢的非血管清除动力学一致。为了验证这些发现,我们使用免疫荧光和成像细胞术对硬脑膜背侧和腹侧进行了高分辨率空间定位,鉴定了沿MMA排列的PROX1+、PDPN+和LYVE1+淋巴样结构。这些发现确定了人类硬脑膜腹侧一个以前未被识别的流出区,并支持在背室之外存在有组织的脑膜淋巴结构。这种综合的人体框架激发了腹侧淋巴管在脑液清除和神经免疫调节中的作用的未来机制研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
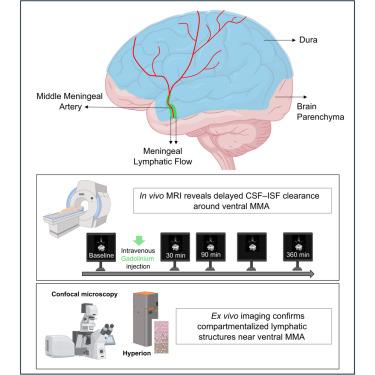
Meningeal lymphatic architecture and drainage dynamics surrounding the human middle meningeal artery
The anatomical basis of cerebrospinal and interstitial fluid clearance along the human ventral meninges remains poorly defined. Here, we present the in vivo and ex vivo evidence of a distinct CSF-ISF drainage compartment surrounding the middle meningeal artery (MMA) in humans. In five healthy participants, dynamic contrast-enhanced MRI revealed delayed signal enhancement along the MMA-peripheral region, peaking at 90 min, substantially later than adjacent vascular and lymphatic structures, consistent with slower, nonvascular clearance dynamics. To validate these findings, we performed the high-resolution spatial mapping of the dorsal and ventral dura using immunofluorescence and imaging mass cytometry, identifying PROX1+, PDPN+, and LYVE1+ lymphatic-like structures that are aligned along the MMA. These findings define a previously unrecognized outflow zone in the human ventral dura and support the presence of organized meningeal lymphatic architecture beyond dorsal compartments. This integrated human framework motivates future mechanistic studies into the role of ventral lymphatics in brain fluid clearance and neuroimmune regulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


