提高碱水电解析氢反应中raney - ni基电极的耐久性:使用NiP保护层减轻逆流和H2泡效应
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Raney Ni (R-Ni)电极在碱性电解(AWE)中用作析氢反应催化剂。然而,由于反向电流诱导的氧化和H2气泡的催化剂损伤,它们不耐用。反向电流触发Ni相变和机械应力,导致催化剂分层,而气泡阻塞活性位点,增加电阻,造成结构破坏。这些问题是单独解决的,但不是同时解决的。在本研究中,在R-Ni电极上电镀p掺杂Ni (NiP)保护层来克服这两个挑战。NiP保护层抑制氧化,减少Ni相变,防止催化剂脱层。增强的表面润湿性最大限度地减少成核,促进更快的气泡脱离,减少气泡相关的损害。电化学测试表明,在−400 mA cm−2时,NiP/R-Ni的过电位比R-Ni低26 mV,具有更高的催化活性。加速降解实验(ADTs)表明,NiP/R-Ni催化剂层保留,ADT后过电位仅增加25 mV,明显小于R-Ni。实时阻抗分析显示,NiP/R-Ni表面存在快速分离的小气泡。总的来说,R-Ni上的NiP保护层同时减轻了逆流和H2气泡引起的降解,提高了AWE期间的催化活性和耐久性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
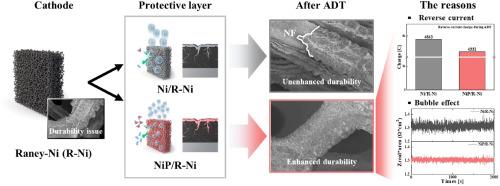
Enhancing durability of Raney-Ni-based electrodes for hydrogen evolution reaction in alkaline water electrolysis: Mitigating reverse current and H2 bubble effects using a NiP protective layer
Raney Ni (R-Ni) electrodes are used as hydrogen evolution reaction catalysts in alkaline water electrolysis (AWE). However, they are not durable because of reverse current-induced oxidation and catalyst damage from H2 bubbles. Reverse current triggers Ni phase changes and mechanical stress, leading to catalyst delamination, while bubbles block active sites, increase resistance, and cause structural damage. These issues have been addressed individually but not simultaneously. In this study, a P-doped Ni (NiP) protective layer is electroplated on the R-Ni electrode to overcome both challenges. The NiP protective layer inhibits oxidation, reducing Ni phase changes and preventing catalyst delamination. Enhanced surface wettability minimizes nucleation and facilitates faster bubble detachment, reducing bubble-related damage. Electrochemical tests reveal that NiP/R-Ni exhibits a 26 mV lower overpotential than that of R-Ni at −400 mA cm−2, indicating higher catalytic activity. Accelerated degradation tests (ADTs) demonstrate the retention of the NiP/R-Ni catalyst layer, with only a 25 mV increase in overpotential after ADT, which is significantly less than that of R-Ni. Real-time impedance analysis reveals the presence of small, rapidly detaching bubbles on NiP/R-Ni. Overall, the NiP protective layer on R-Ni simultaneously mitigates both reverse current and H2 bubble-induced degradation, improving catalytic activity and durability during AWE.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


