二氯仲胺H-N和H-CCl2氢原子对镓(III)的螯合萃取
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
摘要
合成了N-(2-乙基己基)单氯乙酰胺(MCAA)、N-(2-乙基己基)二氯乙酰胺(DCAA)和N-(2-乙基己基)-2,2,2-三氯乙酰胺(TCAA) 3种不同氯原子数的仲酰胺,用于Ga(III)的提取。在较高的HCl浓度下,与其他金属相比,这三种酰胺更倾向于提取Ga(III),并通过斜率分析研究了三种试剂对Ga(III)提取的化学计量学。通过光谱研究阐明了GaCl4−的萃取机理。在较低HCl浓度([HCl] < 8.46 mol dm−3)的介质中,酰胺的(O)=C氧原子没有发生水合氢离子的萃取,说明(H)-N氢原子与GaCl4−表现出明显的相互作用。DCAA对Ga(III)的提取效率最高。质子核磁共振波谱分析和密度泛函理论计算表明,DCAA (H)-C-Cl2中氢原子的δ+值增强,有助于(H)-N氢原子对GaCl4−的萃取。(H)-N氢与部分带负电的(O)=C氧原子之间的稳定的分子间氢键抑制了GaCl4−的提取。用水可使Ga(III)从负载Ga的MCAA和TCAA中完全剥离,而DCAA的螯合萃取抑制了Ga(III)用水剥离的效率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
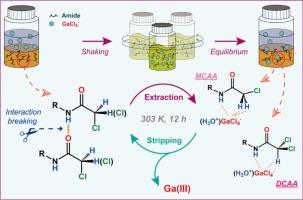
Specific chelating extraction of gallium(III) with H-N and H-CCl2 hydrogen atoms of dichlorinated secondary amide
Three secondary amides with different numbers of chlorine atom(s)—N-(2-ethylhexyl)monochloroacetamide (MCAA), N-(2-ethylhexyl)dichloroacetamide (DCAA), and N-(2-ethylhexyl)-2,2,2-trichloroacetamide (TCAA)—were synthesised for Ga(III) extraction. These three amides exhibited a preference for extracting Ga(III) compared to other metals at higher HCl concentrations, with the stoichiometries of Ga(III) extraction using the three reagents investigated via slope analysis. Spectroscopic studies were performed to elucidate the extraction mechanism of GaCl4−. The hydronium ion extraction with (O)=C oxygen atoms of amides did not occur in lower HCl concentration media ([HCl] < 8.46 mol dm−3), indicating that (H)-N hydrogen atom displayed a distinctive interaction with GaCl4−. The DCAA exhibited the highest Ga(III) extraction efficiency among the three secondary amides. The proton nuclear magnetic resonance spectroscopic analysis and density functional theory calculations indicated that the δ+ value of the hydrogen atom in (H)-C-Cl2 of DCAA was enhanced to assist GaCl4− extraction with the (H)-N hydrogen atom. Stable intermolecular hydrogen bonding between (H)-N hydrogen and the partially negatively charged (O)=C oxygen atoms of MCAA inhibited GaCl4− extraction. The complete stripping of Ga(III) from the Ga-loaded MCAA and TCAA was achieved using water, while chelation extraction with DCAA suppressed the efficiency of Ga(III) stripping with water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


