分子结构对羧酸胆碱离子液体理化性质的影响:实验与理论结合的研究
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
羧酸胆碱离子液体(ILs)是一类生物基质子离子液体,是不溶性天然产物的优良溶剂。本文采用一步法合成了4个羧酸胆碱类化合物;物理化学性质,如密度、表面张力和离子电导率被测量为温度的函数。此外,还计算了实验中难以获得的热膨胀系数、分子体积、标准熵、晶格能和极性等理化参数。为了更好地了解胆碱羧酸盐il的分子间相互作用,利用cosmos - rs测定了胆碱阳离子和羧酸阴离子的sigma谱。结果表明,胆碱阳离子具有作为氢键供体的潜力,而羧酸阴离子具有作为氢键受体的显著能力。通过DFT计算,研究了胆碱阳离子与羧酸阴离子之间可能的相互作用。所有计算表明,胆碱阳离子倾向于通过O- h··O相互作用与羧酸阴离子连接。利用还原密度梯度(RDG)等面进一步分析了胆碱基il中的非共价相互作用。结果表明,丁酸阴离子的o原子与胆碱阳离子的OH之间的氢键相互作用最强。本文章由计算机程序翻译,如有差异,请以英文原文为准。
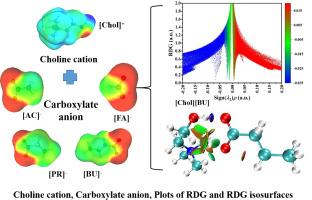
Influence of the molecular structure on physicochemical properties of choline carboxylate ionic liquids: A combined experimental and theoretical study
Choline carboxylate ionic liquids (ILs), which act as a type of bio-based protic ionic liquid (PIL), are excellent solvents for insoluble natural products. In this work, four choline carboxylate ILs were synthesized by a one-step method; physicochemical properties such as density, surface tension, and ionic conductivity were measured as a function of temperature. Additionally, physico-chemical parameters that are difficult to obtain through experiments, including thermal expansion coefficients, molecular volumes, standard entropy, lattice energy and polarity of the ILs were further calculated. To better understand intermolecular interactions of choline carboxylate ILs, the sigma profile of choline cation and carboxylate anion was determined using COSMO-RS. The results showed that the choline cation has potential as an H-bond donor, while the carboxylate anion demonstrates significant capability as a hydrogen bond acceptor. By DFT calculations, the possible interactions between the choline cation and carboxylate anions were investigated. All calculations indicate that the choline cation prefers to connect to the carboxylate anion through O-H···O interactions. Non-covalent interactions in choline-based ILs were further analyzed using reduced density gradient (RDG) isosurfaces. The results show that the hydrogen bond interaction between the O-atom of butyric acid anion and O![]() H of choline cation is the strongest.
H of choline cation is the strongest.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


