镍催化的Chan-Lam偶联:环境条件下n -芳基化2-氨基苯并噻唑的有效途径
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在温和的反应条件下,镍催化2-氨基苯并噻唑和芳基硼酸的C-N偶联。在露天条件下,在Ni/4,4 ' -dOMebpy催化体系的存在下,得到n-芳基化的2-氨基苯并噻唑。该方法涵盖了广泛的适用底物,包括芳基硼酸和2-氨基苯并噻唑,在短的反应时间内以中等到良好的产率提供相应的C-N偶联产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
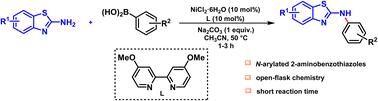
Nickel-catalyzed Chan–Lam coupling: an efficient route to N-arylated 2-aminobenzothiazoles under ambient conditions
A nickel-catalyzed C–N coupling of 2-aminobenzothiazoles and aryl boronic acids under mild reaction conditions is disclosed. The reaction afforded N-arylated 2-aminobenzothiazoles in the presence of a Ni/4,4′-dOMebpy catalytic system under open-air conditions. The method encompasses a wide range of applicable substrates, including aryl boronic acids and 2-aminobenzothiazoles, affording the corresponding C–N coupled products in moderate to good yields in short reaction times.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


