醋酸铜介导的2-吡啶取代丙烯腈与DMSO的硫甲基化反应
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以丙烯腈和二甲亚砜为原料,高效合成了多种烯基甲基硫醚。这种醋酸铜介导的硫甲基化反应提供了相应的产物,具有广泛的底物范围和中等至优异的收率。这种转变是通过乙烯基C-H键的直接功能化实现的,从而形成立体定向的内炔的正规氰基硫代化产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
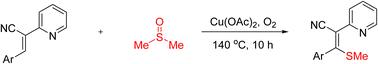
Copper acetate mediated thiomethylation of 2-pyridine-substituted acrylonitriles with DMSO
An efficient synthesis of a variety of alkenyl methyl thioethers from acrylonitriles and dimethyl sulfoxide is described. This copper acetate mediated thiomethylation reaction provides the corresponding products with broad substrate scope in moderate to excellent yields. This transformation is achieved through direct functionalization of vinylic C–H bonds, resulting in stereospecific formation of the formal cyanothiolation product of internal alkynes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


