用TG-DSC研究不同升温速率下麦秸的燃烧行为
IF 5.8
2区 生物学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
摘要
生物质废弃物的燃烧由于其潜在的能源生产潜力和在解决生物质废弃物积累引起的环境问题方面的作用而成为一个热点研究课题。本文利用热重分析-差示扫描量热法研究了麦秸在1073 K范围内不同加热速率下的燃烧。通过热重分析,将燃烧过程分为5个不同的阶段:水分蒸发、挥发分燃烧、木质素分解、残余挥发分和固定碳燃烧以及最后燃尽阶段。随着升温速率的增加,燃烧特征温度、最大燃烧速率和综合可燃性指数均升高。差示扫描量热分析揭示了所有加热速率下的放热反应,并确定了三个放热峰。利用Kissinger-Akahira-Sunose (KAS)法计算得到了一次燃烧相的活化能为121.4±3.7 ~ 316.5±7.9 kJ/mol,随着燃烧的进行,活化能呈增大趋势。指数前因子随升温速率变化,变化范围为1.08 × 109 ~ 2.46 × 1027 s−1。基于活化配合物理论,推导了反应物在燃烧过程中转化为活化配合物的热力学参数。活化焓变化范围为117.0 ~ 310.6 kJ/mol,而活化吉布斯自由能变化平均为162.7 kJ/mol,证实了麦秸降解过程中活化配合物的形成是非自发过程。激活熵变化范围为- 85 ~ 264 J/(mol·K),反映了分子的无序性和能量效率的变化。这些结果为进一步发展农业生物质利用提供了有价值的理论见解和数据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
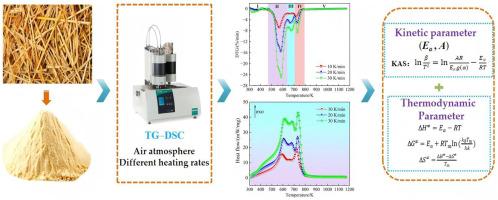
Study of the combustion behavior of wheat straw using TG–DSC under multiple heating rates
The combustion of biomass waste has become a hot research topic due to its potential for energy production and its role in addressing environmental issues caused by biomass waste accumulation. This article investigates the combustion of wheat straw using thermogravimetric analysis-differential scanning calorimetry within a wide range of temperatures up to 1073 K at various heating rates. Based on thermogravimetric analysis, the combustion process is categorized into five distinct phases: moisture evaporation, volatile matter combustion, lignin breakdown, the combustion of residual volatiles and fixed carbon, and the final burnout phase. It was observed that the combustion characteristic temperatures, maximum combustion rate, and comprehensive combustibility index all rise as the heating rate increases. Differential scanning calorimetry analysis revealed exothermic reactions for all heating rates, with three exothermic peaks identified. The activation energy of the primary combustion phase using the Kissinger–Akahira–Sunose (KAS) method was calculated as 121.4 ± 3.7 to 316.5 ± 7.9 kJ/mol, with an increasing trend as combustion proceeds. The pre-exponential factor ranged from 1.08 × 109 to 2.46 × 1027 s−1 for KAS, varying with the heating rate. Based on the activated complex theory, the thermodynamic parameters for the conversion of reactants to the activated complex during combustion were derived. The activation enthalpy change ranges from 117.0 to 310.6 kJ/mol, while the activation Gibbs free energy change, with an average of 162.7 kJ/mol, confirms that activated complex formation during wheat straw degradation is a non-spontaneous process. The activation entropy change spans from −85 to 264 J/(mol·K), indicating molecular disorder and variations in energy efficiency. These results offer valuable theoretical insights and data for the further development of agricultural biomass utilization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomass & Bioenergy
工程技术-能源与燃料
CiteScore
11.50
自引率
3.30%
发文量
258
审稿时长
60 days
期刊介绍:
Biomass & Bioenergy is an international journal publishing original research papers and short communications, review articles and case studies on biological resources, chemical and biological processes, and biomass products for new renewable sources of energy and materials.
The scope of the journal extends to the environmental, management and economic aspects of biomass and bioenergy.
Key areas covered by the journal:
• Biomass: sources, energy crop production processes, genetic improvements, composition. Please note that research on these biomass subjects must be linked directly to bioenergy generation.
• Biological Residues: residues/rests from agricultural production, forestry and plantations (palm, sugar etc), processing industries, and municipal sources (MSW). Papers on the use of biomass residues through innovative processes/technological novelty and/or consideration of feedstock/system sustainability (or unsustainability) are welcomed. However waste treatment processes and pollution control or mitigation which are only tangentially related to bioenergy are not in the scope of the journal, as they are more suited to publications in the environmental arena. Papers that describe conventional waste streams (ie well described in existing literature) that do not empirically address ''new'' added value from the process are not suitable for submission to the journal.
• Bioenergy Processes: fermentations, thermochemical conversions, liquid and gaseous fuels, and petrochemical substitutes
• Bioenergy Utilization: direct combustion, gasification, electricity production, chemical processes, and by-product remediation
• Biomass and the Environment: carbon cycle, the net energy efficiency of bioenergy systems, assessment of sustainability, and biodiversity issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


