纳米塑料诱导的NAT10/ac4C轴驱动氧化应激和化学抗性
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
纳米塑料毒性的确切分子机制仍然知之甚少。本研究首次证明了聚苯乙烯纳米颗粒(PS-NPs)诱导了显著的外转录组重编程,通过衍生化- lc -MS/MS方法检测了38种不同的tRNA单核苷酸和49种寡核苷酸。PS-NPs诱导强氧化应激,活性氧(ROS)增加3.1倍,RNA损伤标志物8-氧- gmp增加2.6倍。此外,这种胁迫上调了乙酰转移酶NAT10,导致纳米塑料胁迫下tRNALeu d环内特异性发生n4 -乙酰胞苷(ac4C)超修饰。有趣的是,NAT10/ac4C轴激活也可以降低对化疗药物索拉非尼的敏感性,将其IC50从6.9 μM提高到25.7 μM。至关重要的是,这种化学耐药通过药物抑制(用重塑蛋白)和基因敲低(用siRNA)逆转,随后改善氧化应激并使细胞对索拉非尼重新敏感,证实了该途径在调节细胞对纳米塑料暴露的反应中的因果作用。我们的研究结果表明,NAT10/ac4C轴的上调是一种针对纳米塑料诱导的应激的靶向性适应性反应,揭示了环境污染物与化疗效果受损之间的直接机制联系。这表明NAT10/ac4C轴既是纳米塑料毒性的关键介质,也是恢复细胞健康的有希望的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
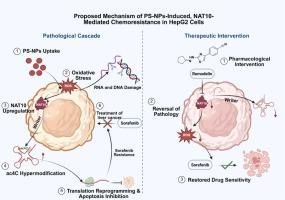
Nanoplastic-Induced NAT10/ac4C Axis Drives Both Oxidative Stress and Chemoresistance
The exact molecular mechanisms of nanoplastics toxicity remain poorly understood. This study provides the first evidence that exposure to polystyrene nanoparticles (PS-NPs) induces a significant epitranscriptomic reprogramming, detecting 38 different tRNA mononucleotides and 49 oligonucleotides through a derivatization-LC-MS/MS approach. PS-NPs induced potent oxidative stress, marked by a 3.1-fold increase in reactive oxygen species (ROS) and a 2.6-fold increase in the RNA damage marker 8-oxo-GMP. Furthermore, this stress upregulated the acetyltransferase NAT10, leading to N4-acetylcytidine (ac4C) hypermodification that occurred specifically within the D-loop of tRNALeu under nanoplastics stress. Interestingly, NAT10/ac4C axis activation could also decrease the sensitivity to the chemotherapeutic agent sorafenib, increasing its IC50 from 6.9 μM to 25.7 μM. Crucially, this chemoresistance was reversed by both pharmacological inhibition (with Remodelin) and genetic knockdown (with siRNA) of NAT10, which subsequently ameliorated oxidative stress and re-sensitized the cells to sorafenib, confirming the pathway's causal role in modulating cellular response to nanoplastic exposure. Our findings establish the upregulation of the NAT10/ac4C axis as a targeted, adaptive response to nanoplastics-induced stress, revealing a direct mechanistic link between an environmental pollutant and impaired chemotherapeutic efficacy. This identifies the NAT10/ac4C axis as both a key mediator of nanoplastics toxicity and a promising therapeutic target to restore cellular health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


