不对称氮杂丁C-H硼化和氧化开环组装无环全碳季位中心
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文报道了一锅法不对称合成无环全碳季立体中心的方法。铱催化3,3-二取代氮杂啶的C(sp3) -H硼化反应,随后氧化开环,得到了各种光学活性的α,α-二取代β-甲酰基酰胺。获得的产物可以进行一系列的下游转化,包括通过插入1-3个碳单元来合成5- 7元饱和氮杂环的正式不对称分子编辑方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
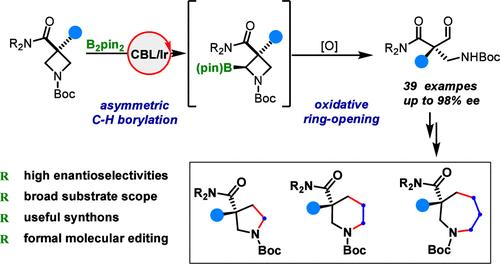
Assembly of Acyclic All-Carbon Quaternary Stereocenters via Asymmetric Azetidine C–H Borylation and Oxidative Ring-Opening
Reported herein is a one-pot protocol for the asymmetric synthesis of acyclic all-carbon quaternary stereocenters. Iridium-catalyzed enantioselective C(sp3)–H borylation of 3,3-disubstituted azetidines followed by oxidative ring-opening afforded a variety of optically active α,α-disubstituted β-formyl amides. The obtained products could undergo a wide array of downstream transformations, including a formal asymmetric molecular editing approach for the synthesis of 5- to 7-membered saturated azacycles via the insertion of 1–3 carbon units.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


