BaZr0.625Ce0.2Y0.175O3-δ基质子导电陶瓷电池的电化学表征及模型研究
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
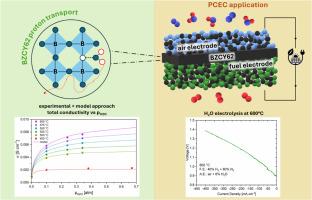
本文研究了钙钛矿氧化物BaZr0.625Ce0.2Y0.175O3-δ (BZCY62)作为质子导电陶瓷电解电池(PCECs)电解液的电化学性能。为了研究导电行为,在400°C至600°C的不同摩尔分数O2(21-2%)、H2O(35-0.1%)和H2下进行了电化学阻抗谱测量。水对BZCY62电导率的有利影响(在600℃时,从0.1% H2O下的0.9 mS cm−1到35% H2O下的7.6 mS cm−1)与饱和阈值的出现一起出现。建立了一个平衡模型来预测主要载流子(质子、极化子和氧空位)的电导率。该模型包括水化、极化子形成和与H2相互作用的消耗反应,并允许分析电导率实验并提取相关热力学和输运参数,得到600℃下2.12 × 10−6 cm2 s−1的质子扩散系数。要确定BZCY62的水化性能,需要综合热重分析的结果。首次制造了基于BZCY62的电解质支持和电极支持按钮pcec,并在500°C至600°C的蒸汽电解中进行了测试。pcec分别安装了Ni-BZCY62燃料电极和BLC-BZCY62 (Ba0.5La0.5CoO3-δ)氧气电极,并在蒸汽/空气混合物浓度为30/70 mol/mol和H2/N2混合物浓度为50/50 mol/mol的情况下进行了电流/电压和阻抗曲线测试。电极支撑的PCEC在1.4 V和600°C下,在6%的加湿空气中达到413 mA cm−2。观察到BZCY62的p型电导率对OCV和电池性能的显著影响是电解质厚度的函数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrochemical characterization and model investigation of BaZr0.625Ce0.2Y0.175O3-δ based proton conductive ceramic cells
In this work, we study the electrochemical properties of BaZr0.625Ce0.2Y0.175O3-δ (BZCY62), a perovskite oxide applied as electrolyte of proton-conductive ceramic electrolysis cells (PCECs). To investigate the conductivity behavior, electrochemical impedance spectroscopy measurements were performed between 400°C and 600°C at varying molar fractions of O2 (21-2%), H2O (35-0.1%), and H2. The beneficial effect of H2O on the BZCY62 conductivity emerged (at 600°C, from 0.9 mS cm−1 at 0.1% H2O to 7.6 mS cm−1 at 35% H2O) together with the occurrence of a saturation threshold. An equilibrium model was developed to predict the conductivities of the main charge carriers (protons, polarons, and oxygen vacancies). The model included reactions of hydration, polaron formation and consumption via interaction with H2, and allowed to analyze the conductivity experiments and extract relevant thermodynamic and transport parameters, finding 2.12 × 10−6 cm2 s−1 protons diffusivity at 600°C. Integration of the results of thermogravimetric analyses was required to determine the hydration properties of BZCY62. Electrolyte-supported and electrode-supported button PCECs based on BZCY62 were manufactured for the first time, and tested in steam electrolysis between 500°C and 600°C. The PCECs mounted Ni-BZCY62 fuel electrodes, and BLC-BZCY62 (Ba0.5La0.5CoO3-δ) oxygen electrodes, and were tested with current/voltage and impedance curves by supplying steam/air mixtures up to 30/70 mol/mol and H2/N2 mixtures up to 50/50 mol/mol. The electrode-supported PCEC reached 413 mA cm−2 at 1.4 V and 600°C, in 6% humidified air. Significant impact of the adverse p-type conductivity of BZCY62 on OCV and cell’s performance as a function of the electrolyte thickness was observed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


