基于量热法和表征方法的NMC811/LLZO/Li固态电池早期热安全性评价
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
随着包括锂金属固态电池在内的各种电池化学物质的出现,早期安全评估可以为安全材料组、电池和电池组的设计提供见解。差示扫描量热法(DSC)可用于测定小(10mg)样品的热流。我们构建了密封的Li/LLZO/NMC811阳极-阴极-电解质(ACE)固态样品进行DSC测试,并测量了放热反应的开始温度、热流速率和500°C时释放的总热量。提出的化学反应途径是利用DSC和TGA的质能平衡,以及其他表征方法的额外输入来构建的。通过在DSC测试(在我们的方案中为500°C)的恒速加热结束时添加等温保持,我们获得了对确定反应途径很重要的额外热流。我们发现我们的NMC811/LLZO/Li ACE样品在500°C时的总放热量为~ 15 J/mAh,起始温度约为220°C。我们将固态材料组与锂金属阳极和LLZO分离器以及NMC811或锂钴氧化物阴极进行了比较,发现热指标(如总放热和起始温度)大致相似,反映了一个共同的放热反应路径,包括层状氧化物阴极活性材料释放氧和PVDF粘合剂释放HF以及它们与还原剂(包括锂金属和导电碳)的反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
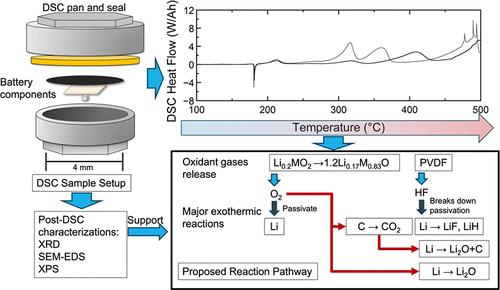
Early-Stage Thermal Safety Evaluation of the NMC811/LLZO/Li Solid-State Battery Chemistry Using Calorimetry and Characterization Methods
With the emergence of various battery chemistries including lithium metal solid-state batteries, early-stage safety evaluations can provide insights into designs for safe material sets, cells, and packs. Differential scanning calorimetry (DSC) can be performed to determine the heat flow of small (<10 mg) samples. We construct hermetically sealed Li/LLZO/NMC811 anode–cathode–electrolyte (ACE) solid-state samples for DSC tests and measure the onset temperature of exothermic reactions, the rate of heat flow, and the total heat released by 500 °C. Proposed chemical reaction pathways are constructed using a mass–energy balance from DSC and TGA, with additional input from other characterization methods. By adding an isothermal hold at the end of the constant rate heating in DSC tests (500 °C in our protocol), we obtain additional heat flow important for determining the reaction pathways. We find that our NMC811/LLZO/Li ACE samples have a total heat release of ∼15 J/mAh at 500 °C with an onset temperature of around 220 °C. We compare solid-state material sets with a Li metal anode and LLZO separator and either NMC811 or lithium cobalt oxide cathode and find that thermal metrics such as the total heat release and the onset temperatures are broadly similar, reflecting a common exothermic reaction path involving oxygen release from layered oxide cathode active materials and HF release from the PVDF binder and their reaction with reductants including lithium metal and conductive carbon.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


