二氢睾酮衍生的二茂铁类固醇偶联物对可变雄激素依赖的人癌细胞系的ros介导的抗增殖作用
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
二茂铁附着物通常赋予有机支架活性氧(ROS)介导的细胞毒性,但二茂铁-雄激素偶联物的研究仍然很少,特别是那些连接在类固醇a环上的,已知可调节雄激素受体(AR)结合的偶联物。合成了三种c -2取代二茂铁甾体偶联物,这些偶联物是由最有效的人类雄激素二氢睾酮(DHT)衍生的;单晶x射线晶体学证实了1的结构。在ar阳性(LNCaP, OVCAR-3)和ar阴性(PC-3)癌细胞和非恶性MRC-5成纤维细胞中量化抗增殖活性。二茂铁的存在所带来的雄激素原性和固有细胞毒性的平衡被证明是充分的,因为所有的偶联物对两种激素应答的癌细胞系都有抗增殖的作用。偶联物2 (2α-二茂铁甲基dht)抑制OVCAR-3生长的IC50为2.8 μM,相对于顺铂的选择性指数为3.0,是最有效的类似物。在OVCAR-3细胞中,2触发s期阻滞,细胞内铁增加21倍,ros依赖性生存能力丧失;与n -乙酰-l-半胱氨酸共同处理,而不是caspase抑制剂Ac-DEVD-CHO,挽救了细胞。在多细胞肿瘤球体中,2个球体完整性被破坏(IC50 = 14 μM)。这些发现表明,a环取代雄激素-二茂铁偶联物作为激素依赖性癌症的抗增殖药物的潜力,其中2作为一种有希望的候选药物,其效力超过顺铂,并似乎通过独特的机制起作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
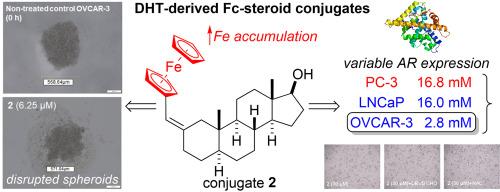
ROS-mediated antiproliferative effects of dihydrotestosterone-derived ferrocene–steroid conjugates toward human cancer cell lines of variable androgen dependence
Ferrocene appendages often endow organic scaffolds with reactive-oxygen-species (ROS)-mediated cytotoxicity, yet ferrocene–androgen conjugates remain somewhat poorly explored, especially those linked at the steroid A-ring, known to modulate androgen receptor (AR) binding. Three C-2-substituted ferrocene–steroid conjugates derived from dihydrotestosterone (DHT) – the most potent human androgen – were synthesized; the structure of 1 was confirmed by single-crystal X-ray crystallography. Antiproliferative activity was quantified in AR-positive (LNCaP, OVCAR-3) and AR-negative (PC-3) cancer cells and in non-malignant MRC-5 fibroblasts. The balance of androgenicity and inherent cytotoxicity brought about by the presence of ferrocene proved adequate, as all conjugates acted in an antiproliferative manner toward the two hormone-responsive cancerous cell lines. Conjugate 2 (2α-ferrocenylmethyl-DHT) was the most potent analogue, inhibiting OVCAR-3 growth with an IC50 = 2.8 μM and a selectivity index of 3.0 relative to cisplatin. In OVCAR-3 cells, 2 triggered S-phase arrest, a 21-fold rise in intracellular iron, and ROS-dependent loss of viability; co-treatment with N-acetyl-l-cysteine, but not the caspase inhibitor Ac-DEVD-CHO, rescued cells. In multicellular tumor spheroids, 2 disrupted spheroid integrity (IC50 = 14 μM). These findings indicate the potential of A-ring substituted androgen-ferrocene conjugates as antiproliferative agents for hormone-dependent cancers, with 2 emerging as a promising candidate that surpasses cisplatin in potency and appears to act through a distinct mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


