雷帕霉素衍生物作为雷帕霉素和GLS1双重抑制剂的首个哺乳动物靶点的设计、合成和生物学评价
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
哺乳动物雷帕霉素靶蛋白(mTOR)和谷氨酰胺酶1 (GLS1)是调节乳腺癌代谢重编程的关键酶。第一代mTOR和GLS1双抑制剂是在抗癌协同作用的基础上设计合成的。化合物9d对所有乳腺癌细胞系均表现出选择性和强抑制增殖活性,对mTOR (mTORC1和mTORC2)和GLS1均表现出强抑制活性。机制研究表明,9d有效调节与mTOR和GLS1抑制相关的生物标志物和代谢物水平,引发持续和大量活性氧的产生,导致细胞自噬、凋亡和铁死亡。此外,9d还能抑制乳腺癌细胞的转移、侵袭和血管生成。体内实验表明,9d显著抑制肿瘤生长和转移,无明显毒性。这些发现证明了mTOR/GLS1双抑制剂对乳腺癌的巨大治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
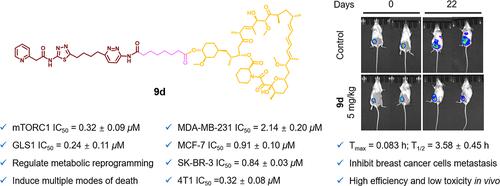
Design, Synthesis, and Biological Evaluation of Rapamycin Derivatives as the First Mammalian Target of Rapamycin and GLS1 Dual Inhibitors
The mammalian target of rapamycin (mTOR) and glutaminase 1 (GLS1) are key enzymes regulating metabolic reprogramming in breast cancer. The first generation of mTOR and GLS1 dual inhibitors was designed and synthesized on the basis of anticancer synergism. Compound 9d showed selective and potent antiproliferative activity against all breast cancer cell lines and displayed potent inhibitory activity against both mTOR (mTORC1 and mTORC2) and GLS1. Mechanism studies revealed that 9d effectively modulated the level of biomarkers and metabolites associated with mTOR and GLS1 inhibition and triggered sustained and massive reactive oxygen species generation, leading to cell death by autophagy, apoptosis, and ferroptosis. Moreover, 9d inhibited the metastasis, invasion, and angiogenesis of breast cancer cells. In vivo experiments demonstrated that 9d significantly inhibited tumor growth and metastasis, without observable toxicity. These findings proved mTOR/GLS1 dual inhibitors’ great therapeutic potential for breast cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


