宿主主导的氧化级联反应和跨代铜转移驱动了慢性CuO纳米颗粒暴露下的穗状夜蛾种群崩溃:纳米农药环境风险评估的意义
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
纳米氧化铜在农业中的使用日益增加,引起了人们对其长期生态风险的迫切关注,特别是在害虫物种的跨代繁殖和肠道微生物群方面。在这里,我们长期暴露于亚致死浓度的CuO NPs(25 mg/kg)中。人口崩溃发生在F4代(0 %生存),由明显的氧化应激(CAT和MDA活动增加了2.0倍),大量铜积累在鸡蛋(12 → 30 ng /毫克)和肠道组织(25 → 750 ng /毫克),和受损的繁殖输出(33 %减少产蛋只有30 %孵化F3)。虽然肠道微生物多样性在结构上保持稳定(Shannon指数,P > 0.05),但宏基因组分析显示功能重编程,尤其是能量代谢。无菌幼虫接种试验证实,微生物群加剧了毒性,尽管直接的NPs效应占主导地位。这些发现突出表明,目前的风险评估框架主要关注急性毒性和微生物组成,严重低估了纳米农药的危害。我们提倡将多代毒性测试和宏基因组分析整合到纳米农药风险评估中,以更好地捕捉种群水平的结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
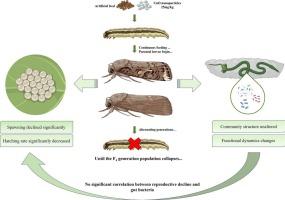
Host-dominated oxidative cascades and transgenerational Cu transfer drive population collapse in Spodoptera frugiperda under chronic CuO nanoparticle exposure: Implications for nano-pesticide environmental risk assessment
The increasing use of CuO nanoparticles (NPs) in agriculture raises urgent concerns about their long-term ecological risks, particularly regarding transgenerational reproduction and gut microbiota in pest species. Here, we chronically exposed Spodoptera frugiperda to a sublethal concentration of CuO NPs (25 mg/kg). Population collapse occurred in the F4 generation (0 % survival), driven by pronounced oxidative stress (CAT and MDA activities increased up to 2.0-fold), substantial Cu accumulation in eggs (12 → 30 ng/mg) and gut tissue (25 → 750 ng/mg), and impaired reproductive output (33 % reduction in egg production with only 30 % hatching in F3). Although gut microbial diversity remained structurally stable (Shannon index, P > 0.05), metagenomic analysis revealed functional reprogramming, particularly in energy metabolism. Sterile larva inoculation assays confirmed that microbiota exacerbated toxicity, though direct NPs effects dominated. These findings highlight that current risk assessment frameworks, which are primarily focused on acute toxicity and microbial composition, severely underestimate the hazards of nanopesticides. We advocate for integrating multigenerational toxicity testing and metagenomic profiling into nano-pesticide risk evaluations to better capture population-level outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


