基于系统生物学的药物重新定位识别细胞外基质模块作为肺鳞状细胞癌的治疗靶点
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
跨癌症亚型的系统蛋白质组学比较提供了对肿瘤异质性的见解,并加速了治疗靶点和药物重新定位的发现。在这里,我们提出了一个新的计算框架,基于特征网络微扰的药物重新定位(SnpDR),通过差异模块分析,药物反应网络构建和多尺度微扰响应扫描整合蛋白质组学和药物基因组学数据。应用snp比较肺腺癌和肺鳞状细胞癌(LSCC)的蛋白质组学景观,我们发现细胞外基质(ECM)模块是LSCC的中心枢纽,而LAMA1则是一个新的药物靶点。体外和体内实验验证了两种靶向ECM成分的重新定位药物Fingolimod和Piperlongumine在浓度低于10 μM时显著抑制LSCC细胞生长、增殖和迁移。这些结果为系统生物学识别亚型特异性治疗脆弱性的能力提供了令人信服的证据。我们的研究结果强调了一个有希望的精准肿瘤学框架,并强调了ecm靶向干预LSCC的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
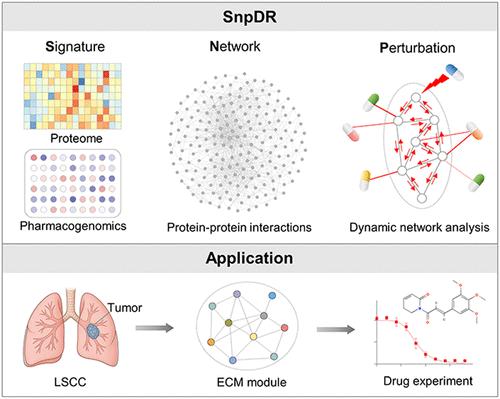
Systems Biology-Based Drug Repositioning Identifies Extracellular Matrix Module as a Therapeutic Target in Lung Squamous Cell Carcinoma
Systematic proteomic comparisons across cancer subtypes provide insights into tumor heterogeneity and accelerate discovery of therapeutic targets and drug repositioning. Here, we present a novel computational framework, signature-network-perturbation- based drug repositioning (SnpDR), integrating proteomic and pharmacogenomic data through differential modular analysis, drug response network construction, and multiscale perturbation response scanning. Applying SnpDR to compare the proteomic landscapes of lung adenocarcinoma and lung squamous cell carcinoma (LSCC), we identified the extracellular matrix (ECM) module as a central hub in LSCC, while LAMA1 emerged as a novel drug target. In vitro and in vivo experiments validated two repositioned drugs, Fingolimod and Piperlongumine, both targeting ECM components, significantly inhibited LSCC cell growth, proliferation and migration at concentrations below 10 μM. These results provide compelling evidence for the power of systems biology to identify subtype-specific therapeutic vulnerabilities. Our findings highlight a promising framework for precision oncology and underscore the potential of ECM-targeted interventions in LSCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


