增加L-SIGN特异性糖模拟物的化学空间
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
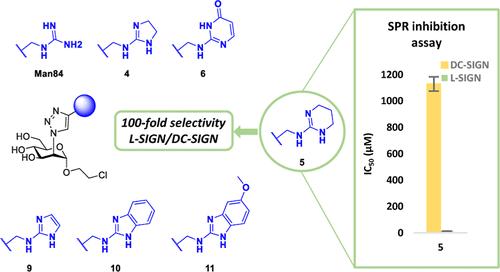
c型凝集素受体L-SIGN的选择性配体为抗病毒治疗和组织特异性递送提供了有希望的途径。我们最近报道了一种含有胍的修饰甘露糖拟糖物Man84,它以微摩尔亲和力和高选择性结合L-SIGN,对抗同系物凝集素DC-SIGN。在这里,我们描述了一系列保持或提高这种选择性的Man84同位体(配体2-11)。利用固定的SARS-CoV-2刺突蛋白,通过表面等离子体共振抑制实验评估了配体对L-SIGN的亲和力以及对DC-SIGN的选择性。化合物4、5和9与L-SIGN的结合具有低的微摩尔亲和力和50 - 94倍的选择性,从而达到或超过了Man84的性能。对L-SIGN CRD/4配合物的晶体结构进行了解析,并强调了配体与L-SIGN中E370侧链的双齿h键相互作用的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Increasing the Chemical Space of L-SIGN Specific Glycomimetics
Selective ligands for the C-type lectin receptor L-SIGN offer promising avenues in antiviral therapies and for tissue-specific delivery. We recently reported that a guanidine-bearing modified mannose glycomimetic, called Man84, binds to L-SIGN with micromolar affinity and high-selectivity against the homologue lectin DC-SIGN. Here we describe a series of Man84 isosteres (ligands 2–11) that maintain or improve on this selectivity. The affinity of the ligands for L-SIGN, as well as their selectivity against DC-SIGN, were evaluated by Surface Plasmon Resonance inhibition assays using immobilized SARS-CoV-2 Spike protein. Compounds 4, 5 and 9 were found to bind to L-SIGN with low micromolar affinity and 50–94-fold selectivity, thus matching or exceeding the performance of Man84. The crystal structure of the L-SIGN CRD/4 complex was solved and highlighted the critical role of a bidentate H-bond interaction of the ligands with the side chain of E370 in L-SIGN.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


