螺旋状银电聚合成导电金属聚合物的结构和功能研究
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
通过与含有两个三苯胺(TPA)的n4给体配体的络合反应,合成了一种新型的发光双核螺旋银(I)配合物。x射线衍射分析证实了该配合物的结构,并揭示了亲银相互作用的存在。光物理研究证实了配体L以及Ag(I)配合物在固态下是发光的。Ag(I)配合物在蓝色区域表现出配体分子的锐利发射带,在475 nm ~ 700 nm范围内表现出受亲银相互作用干扰的配体-金属电荷转移跃迁(LMCT)的宽发射带。自由配体L及其Ag(I)配合物均发生电聚合,从而形成薄膜。此外,与配体l相比,Ag(I)配合物的电聚合更容易发生。所得到的聚合物具有明显的光谱电化学性能,稳定性增强,在氧化还原开关时从黄绿色到橙色到蓝色两步变色,着色/漂白时间快(2.7 s)。这些研究表明,银(I)螺旋配合物可以作为一种有效的电致变色材料,并可用于电致变色器件。Ag(I)离子的存在有利于在电极表面形成薄层聚合物,提高了聚合物的电致变色性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
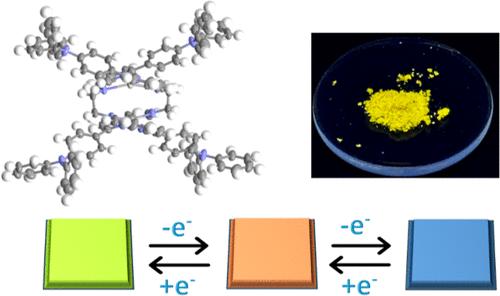
Electropolymerization of Silver(I) Helicate into Conductive Metallopolymer: Structural and Functional Insights
A novel luminescent dinuclear helicate silver(I) complex has been synthesized via a complexation reaction with a N4-donor ligand containing two triphenylamine (TPA) moieties. The structure of the complex has been confirmed by X-ray diffraction analysis and it revealed the presence of argentophilic interactions. The photophysical studies confirmed that ligand L, as well as the Ag(I) complex, is luminescent in the solid state. The Ag(I) complex exhibited a sharp emission band in the blue region from ligand molecules and broad emission band in the range 475 nm–700 nm assigned to ligand-to-metal charge transfer transition (LMCT) perturbed by the presence of argentophilic interactions. Both free ligand L and its Ag(I) complex undergo electropolymerization, leading to the formation of thin films. Moreover, electropolymerization of the Ag(I) complex occurred more easily compared with ligand L. The resulting polymer exhibited distinct spectroelectrochemical properties, enhanced stability, and 2-step color change from greenish yellow via orange to blue upon redox switching with fast coloration/bleaching times (2.7 s). These investigations demonstrate that the silver(I) helicate complex can serve as an effective electrochromic material and can be used in electrochromic devices. It was also shown that the presence of Ag(I) ions facilitates the formation of a thin layer of polymer on the electrode surface and improves the electrochromic performance of the polymer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


