基于酶分析的组织蛋白酶-可切割抗体-药物偶联物-药物手性纯度评价平台
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
控制抗体-药物偶联物(adc)的可切割连接药物的手性纯度是很重要的,但传统的色谱方法受到开发时间长、柱筛选广泛和需要难以合成的杂质标准的阻碍。在这里,我们报告了一种酶消化实验,该实验使用蛋白酶木瓜蛋白酶选择性地消化组织蛋白酶可切割连接药物所需的(S)-瓜氨酸外显体。这种方法允许未消化的(R)-外显体通过标准反相液相色谱(RPLC)作为其可量化的替代物。结果表明,该方法与手性超临界流体色谱法(SFC)具有高度的线性(R2 > 0.999)、特异性和正交性。该分析的分子不可知性通过其成功应用于各种连接体格式(包括Sq-Cit, Val-Cit和GGFG序列)得到证实,而其实际实用性通过区分具有不同外显体水平的生产批次(1.9% vs. 9.9%)得到证实。将该分析与质谱联用进一步将其应用于发色团差的底物。这种适合用途的酶分析提供了一种有价值和有效的分析工具,可以加速对ADC治疗药物开发过程的理解和优化,特别是在工艺开发的早期阶段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
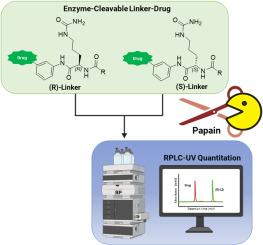
Enzymatic assay based platform for the chiral purity assessment of cathepsin-cleavable antibody-drug conjugate linker-drugs
Controlling the chiral purity of cleavable linker-drugs for antibody-drug conjugates (ADCs) is important, but traditional chromatographic methods are hindered by long development timelines, extensive column screening, and the need for difficult-to-synthesize impurity standards. Here, we report an enzymatic digestion assay that uses the protease papain to selectively digest the desired (S)-citrulline epimer of cathepsin-cleavable linker-drugs. This approach allows the undigested (R)-epimer to serve as its own quantifiable surrogate via standard reversed-phase liquid chromatography (RPLC). Optimized for robustness using Design of Experiments, the assay was shown to be highly linear (R2 > 0.999), specific, and orthogonal to chiral supercritical fluid chromatography (SFC). The molecule-agnostic nature of the assay was confirmed by its successful application to various linker formats, including Sq-Cit, Val-Cit, and GGFG sequences, while its practical utility was demonstrated by distinguishing between manufacturing lots with different epimer levels (1.9 % vs. 9.9 %). Coupling the assay with mass spectrometry further extends its utility to substrates with poor chromophores. This fit-for-purpose enzymatic assay offers a valuable and efficient analytical tool to accelerate process understanding and optimization in the development of ADC therapeutics, particularly at the earliest stages of process development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


